|
Ordering information |
|||
|
Name |
Cat. No. |
Vol. |
Scheme |
|
GS-OH (5 C spacer) |
PMG003-2 PMG003-5 |
2ml 5ml |
|
|
GL-OH (15 C spacer) |
PMG004-2 PMG004-5 |
2ml 5ml |
|
1. Overview
PuriMag™ G-OH Hydroxyl-Functionalized Magnetic Nanoparticles are uniform, polymer-coated superparamagnetic nanoparticles featuring a surface coated with a high density of hydroxyl functional groups. These beads are designed for covalent coupling of carboxyl-containing ligands in organic solvents.
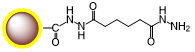
Schematic Diagram of Coupling Mechanism

2. product description
Product Specifications
Description
Polymer coated Fe3O4 nanoparticles
Particle Size
200 nm
Number of Beads
~1.7×1010 beads/mg
Matrix
Proprietary polymer
Functional group
Hydroxy group
Group density
~300 µmole / g of Beads
Magnetization
60~70 EMU/g
Formulation
10 mg/ml suspension in DI water
Stability
pH 3.5~10, 4~80 ℃, most organic solvents
Storage
1 year at 4~8 ℃. Do not freeze.
3. Instructions for Use
A. Required Reagents
N,N-Dimethylformamide (DMF);
Triethylamine;
Methanol;
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC・HCl)
B. Coupling Procedure
1.Dissolve ligand in DMF to prepare 500 μL of 100 mM ligand solution.
2.Aliquot 2.5 mg hydroxyl beads into four 1.5 mL centrifuge tubes.
3.Perform magnetic separation, discard supernatant.
4.Add 500 μL DMF, disperse beads ultrasonically.
5.Perform magnetic separation, discard supernatant.
6.Repeat Steps 4–5 twice (total 3 washes).
7.Add DMF, triethylamine, and prepared ligand solution according to table. Disperse beads ultrasonically.
Concentration (mM)
0
2
10
50
OH beads (mg)
2.5
2.5
2.5
2.5
DMF (μL)
450
440
400
200
100 mM ligand (μL)
0
10
50
250
triethylamine(μL)
50
50
50
50
EDC (mg)
0
1
5
24
Total
500
500
500
500
8.Add 5× molar excess EDC・HCl (e.g., 24 mg for 50 mM ligand). Mix.
*(Alternatively: Pre-weigh EDC・HCl in separate tube before adding ligand/beads)*
9.React at RT for 16–20 h (overnight) with tube mixer.
10.Perform magnetic separation, discard supernatant.
11.Add 500 μL DMF, disperse ultrasonically.
12.Perform magnetic separation, discard supernatant.
13.Repeat Steps 11–12 twice (total 3 washes).
14.Add 500 μL 50% MeOH, disperse ultrasonically.
15.Perform magnetic separation, discard supernatant.
16.Repeat Steps 14–15 twice (total 3 washes).
17.Resuspend in 100 μL 50% MeOH. Store at 4°C.
(Ligand-immobilized bead concentration: 0.5 mg/20 μL)
C. General Affinity Purification Protocol
1.Transfer optimized bead amount to tube. Separate magnetically, discard supernatant.
*Note: Titrate beads against target protein abundance. Typical binding: 1–20 μg target protein per mg beads. Excess beads increase background; insufficient beads reduce yield.*
2.Wash beads 3× with 5 bead volumes PBS (30 sec resuspension per wash).
3.Incubate beads with crude sample containing target protein (1–2 h at RT or optimized temperature; lower temperatures require longer incubation).
4.Wash with PBS or 1M NaCl until eluate OD₂₈₀ < 0.05.
5.Elute target using:
Low pH (2–4)
High pH (10–12)
High salt
Heat
Affinity elution
Boiling in SDS-PAGE loading buffer