Ordering information
Name
Cat. No.
Vol.
Scheme
GS-NH2
(5 C spacer)
PMG005-2
PMG005-5
2ml
5ml
GL-NH2
(15 C spacer)
PMG006-2
PMG006-5
2ml
5ml

1. Overview
PuriMag™ G-NH₂, Amino-Functionalized Magnetic Nanoparticles are uniform, polymer-coated superparamagnetic nanoparticles featuring a surface coated with a high density of amino functional groups. Their hydrophilic surface ensures excellent dispersibility, low non-specific adsorption, and easy handling in various buffers.
The reactive surface amines enable immobilization of ligands such as proteins, peptides, carbohydrates, or other specific molecules. Ligand attachment can be achieved via:
-
Reductive amination with aldehydes/ketones without pre-activating the bead surface, or
-
EDC-mediated crosslinking for ligands containing carboxyl groups.
PuriMag™ G-NH₂ beads can be coupled to peptides, enzymes, carbohydrates, etc., to isolate diverse targets including hormones, receptors, lectins, disease markers, and bacteriophages.
Workflow:
Incubate ligand-conjugated beads with target molecule samples.
Affinity-capture targets via short incubation.
Magnetically separate and remove supernatant.
Wash target-bound beads to obtain purified samples.
Captured targets can be directly used in bioassays or analyzed via SDS-PAGE.
2. product description
|
Product Specifications |
|
|
Description |
Polymer coated Fe3O4 nanoparticles |
|
Particle Size |
200 nm |
|
Number of Beads |
~1.7×1010 beads/mg |
|
Matrix |
Proprietary polymer |
|
Functional group |
Amine group |
|
Group density |
~300 µmole / g of Beads |
|
Magnetization |
60~70 EMU/g |
|
Formulation |
10 mg/ml suspension in DI water |
|
Stability |
pH 3.5~10, 4~80 ℃, most organic solvents |
|
Storage |
1 year at 2~8 ℃. Do not freeze. |
3. Instructions for Use
Washing PuriMag™ Amino Beads:
1.Resuspend beads and transfer required amount to a clean microcentrifuge tube.
2.Separate magnetically, remove supernatant.
3.Add 200 μL coupling buffer, resuspend beads.
4.Separate magnetically, remove supernatant.
5.Repeat wash twice.
Protocol 1: Coupling Aldehydes/Ketones (Reductive Amination)
Ligands with aldehydes/ketones form Schiff's bases with amino beads, reduced by sodium cyanoborohydride.
Buffers:
Coupling Buffer: 0.1 M Sodium Phosphate + 0.15 M NaCl or 100 mM Sodium Borate pH 9.5 or 100 mM Sodium Citrate pH 9.5
Reducing Agent: 5 M Sodium Cyanoborohydride in 1 M NaOH
Quenching Buffer: 0.1 M Ethanolamine, pH 7.4
Procedure:
1.Dissolve ligand in coupling buffer (1–10 mg/mL).
2.Add ligand to washed beads. Mix.
3.Add 1 μL 5M NaCNBH₃ per 100 μL reaction mix. Incubate 2 h at RT. Separate, discard supernatant.
4.Add equal volume quenching buffer. Incubate 15 min at RT. Separate, discard supernatant.
5.Wash 3× with PBS.
Protocol 2: Activation with NHS Crosslinkers
NHS esters react with amines, thiols, carboxyls, hydroxyls, or via photoactivation. Use 10× molar excess crosslinker.
Buffers:
Coupling Buffer: 0.1 M Sodium Phosphate + 0.15 M NaCl, pH 7.4
Quenching Buffer: 0.05 M Tris, pH 7.0
Bead Activation:
1.Resuspend beads in coupling buffer.
*Avoid amine-containing buffers (Tris, glycine).*
2.Add freshly prepared NHS crosslinker solution. Mix.
3.Incubate 30 min at RT. Separate, discard supernatant. Wash 2× with coupling buffer.
Protocol 2-1: Coupling NH₂-Reactive NHS-Esters
For peptides/proteins via N-terminus.
1.Resuspend activated beads. Adjust concentration.
2.Add calculated ligand amount. Mix.
3.Incubate: 30 min RT or 2 h at 4°C.
4.Separate, discard supernatant.
5.Quench with 0.05 M Tris (pH 7.0), 15 min RT.
6.Wash 2× with coupling buffer.
Protocol 2-2: Coupling SH-Reactive NHS-Esters
For thiol-containing ligands (pyridyldithiol, iodo/bromoacetyl, maleimide).
1.Resuspend activated beads in thiol-appropriate buffer.
2.Add free thiol ligand. Vortex.
3.Incubate per manufacturer’s time/temperature.
4.Quench with 5 mM cysteine, 15 min RT (optional).
5.Wash beads.
SH-reactive group
Recommended buffer
Recommended condition
Maleimide
0.1M sodium phosphate pH 6.5-7.5
4 h at 4℃ or 2 h at room temp
Iodo/Bromoacetyl
0.05M sodium borate pH8.3
1h, room temp. Protect from light.
Pyridyldithio
Phosphate buffered saline (PBS) pH7.5
Over night at room temp
Protocol 2-3: Coupling Photo-Reactive NHS-Esters
For aryl azides (hydroxyphenyl, nitrophenyl, perfluorophenyl). Perform in dark.
1.Resuspend activated beads. Adjust concentration.
2.Add ligand. Mix.
3.Irradiate with specified wavelength/time/temp.
4.Optimize: Beads may quench light.
5.Wash 2×.
*Note: Activation/coupling in dry organic solvents (e.g., DMF) reduces hydrolysis. Wash with water before transferring to aqueous buffers.*
Protocol 3: Coupling Carboxyl Ligands Using Amine/Carboxyl-Reactive Crosslinkers
When the ligand contains no other primary amines, EDC or EDC/NHS (or other carbodiimides) can couple carboxyl groups on the ligand to amines on the bead surface. EDC reacts with carboxyl groups to form an amine-reactive intermediate, which is unstable in aqueous solutions. NHS is introduced to stabilize this intermediate.
Buffers:
Coupling Buffer: 0.1 M MES, 0.5 M NaCl, pH 6.0
Reagents: 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC); Sulfo-NHS or N-hydroxysuccinimide (NHS)
Blocking Buffer: 50 mM Tris, pH 8.0 or 5–10 mM Hydroxylamine
Wash Buffer: PBS (pH 7.4)
Note: For EDC coupling, avoid buffers containing free amines or phosphates (e.g., Tris, acetate, glycine), as they reduce efficiency. Thiol-containing buffers irreversibly bind EDC and inhibit coupling.
Procedure for Carboxyl Ligand Coupling:
1.Wash PuriMag™ beads with 0.1 M MES, pH 4.5–5 or 0.1 M MES + 0.5 M NaCl, pH 6.0. Separate magnetically, discard supernatant.
2.Dissolve ligand in the same coupling buffer (1–10 mg/mL). Add the recommended amount to beads. Mix by pipetting.
3.Prepare fresh:
10 mg EDC + 15 mg NHS per mL cold deionized water
10 mg EDC in 1 mL cold deionized water
Keep solutions on ice, protected from light.
4.Add 50–100 μL EDC (or EDC/NHS) solution per mg ligand. Vortex.
5.Incubate 2 h at RT or 2 h at 4°C.
Bead Blocking:
1.Resuspend beads in 500 μL blocking buffer. Incubate 30 min at RT.
2.Separate magnetically, discard supernatant.
3.Repeat blocking step (Steps 1–2).
4.Wash beads 2× with 500 μL PBS (pH 7.4).
5.Resuspend in 100 μL PBS (pH 7.4). Store at 4°C.
Protocol 4: Protein or Amine Ligand Coupling via Glutaraldehyde-Activated Beads
1. Bead Activation:
a) Transfer 0.1 mL magnetic beads (10 mg/mL) to a 2 mL microcentrifuge tube. Perform magnetic separation. Wash 3× with 1 mL PBS (0.02 M, pH 7.0).
b) Disperse beads in 1 mL PBS (0.02 M, pH 7.0). Add 0.05 mL of 25% (w/v) glutaraldehyde solution. Activate at 25°C for 12 h.
c) Perform magnetic separation. Wash 3× with 1 mL PBS (0.02 M, pH 7.0) using ultrasonication to remove residual glutaraldehyde. Resuspend activated beads in 1 mL PBS (0.02 M, pH 7.0) to achieve 1 mg/mL bead suspension.
2. Protein Immobilization:
a) Transfer 1 mL activated beads (1 mg/mL) to a 2 mL tube. Add 0.1 mL target protein (~4 mg/mL). React with rotation at 25°C for 4 h.
b) Perform magnetic separation, discard supernatant. Wash 3× with 1 mL PBS (0.02 M, pH 7.0).
c) Disperse immobilized protein in 5 mL PBS (0.02 M, pH 7.0) to achieve 1 mg/mL concentration. Store at 4°C.
Note: The PBS buffer used in reactions and storage may be substituted with any buffer that maintains optimal activity of the target protein, provided its concentration remains consistent.
Appendix: Immobilization of Carboxyl-Containing Small Molecules onto Amino Beads in Organic Phase (NHS Method)
For screening purposes, initial optimization of ligand loading density on the beads is essential. Varying ligand concentration allows control over immobilization density. This protocol demonstrates ligand immobilization at four concentrations (0 mM, 0.4 mM, 2 mM, and 10 mM).
1. Materials
1.1 Beads & Ligand
PuriMag™ G-NH₂ Beads: 10 mg (Functional group density: ~200 nmol/mg)
Ligand: ~5 mg
1.2 Reagents
N,N-Dimethylformamide (DMF): 25 mL
N-Hydroxysuccinimide (HOSu), M.W. 115.09: 5 mg
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), M.W. 155.24: 5 mg
Triethylamine: 200 μL
Acetic anhydride, M.W. 102.09: 80 μL
Methanol (MeOH): 5 mL
1.3 Equipment
Centrifuge, Vortex Mixer, Rotating Mixer, Ultrasonic Disperser, Magnetic Stand
2. Method
2.1 Mechanism
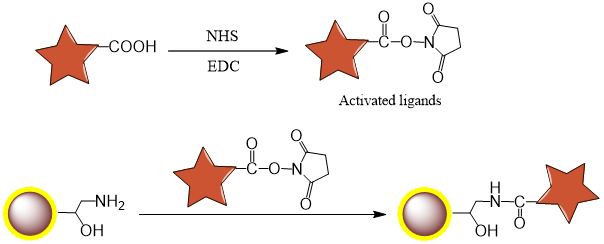
2.2 Procedure
1.Dissolve ligand in DMF to prepare 500 μL of 20 mM solution (final concentration: 10 mM).
2.Prepare 100 μL of 200 mM NHS solution in DMF.
3.Prepare 100 μL of 200 mM EDC solution in DMF.
4.Mix:
400 μL DMF
50 μL 200 mM NHS
50 μL 200 mM EDC
500 μL ligand solution (20 mM)
Vortex at RT for 2 h (equimolar reaction).
20 mM ligand (compound) (μL)
500 (10μmol)
200mM succinimide (μL)
50 (10μmol)
200mM EDC (μL)
50 (10μmol)
DMF (μL)
400
Total (μL)
1000
5.Aliquot 2.5 mg NH₂ beads into four 1.5 mL centrifuge tubes.
6.Magnetically separate at RT, discard supernatant.
7.Add 500 μL DMF, disperse beads ultrasonically.
8.Magnetically separate at RT, discard supernatant.
9.Repeat Steps 7–8 twice (total 3 washes).
10.Add DMF to each tube for dilution.
11.Add activated ligand solution (10 mM final), disperse ultrasonically.
*Note: Add ligand solution after DMF to prevent localized high concentration.*
12.Incubate with vortex mixing at RT for 16–20 h.
Concentration (mM)
0
0.4
2
10
NH2 beads (mg)
2.5
2.5
2.5
2.5
DMFμL
500
480
400
0
Activated 10 mM ligand (μL)
0
20
100
500
Total (μL)
500
500
500
500
13.Magnetically separate at RT, discard supernatant.
14.Add 500 μL DMF, disperse ultrasonically.
15.Magnetically separate at RT, discard supernatant.
16.Repeat Steps 14–15 twice (total 3 washes).
17.Resuspend in 430 μL DMF via ultrasonication.
18.Add 50 μL triethylamine + 20 μL acetic anhydride (0.2 mmol).
19.Vortex at RT for 2 h (blocks unreacted amines).
20.Magnetically separate at RT, discard supernatant.
21.Add 500 μL DMF, disperse ultrasonically.
22.Magnetically separate at RT, discard supernatant.
23.Repeat Steps 21–22 twice (total 3 washes).
If ligand contains OH groups (risk of acetylation):Deacetylate in 500 μL 0.1 M NaOH, 30 min RT.Wash 3× with 500 μL ultrapure water (centrifugation/ultrasonication).
24.Add 500 μL 50% MeOH, disperse ultrasonically.
25.Magnetically separate at RT, discard supernatant.
26.Repeat Steps 24–25 twice (total 3 washes).
27Resuspend in 100 μL 50% MeOH. Store at 4°C.
*Ligand-immobilized bead concentration: 0.5 mg/20 μL*