|
Ordering information |
|||
|
Name |
Cat. No. |
Vol. |
Scheme |
|
G-N3 |
PMG012-2 |
2 ml |
|
1. Overview
Click Chemistry describes rapid, selective "click" reactions between paired functional groups in mild aqueous solutions. This concept has evolved into a convenient, versatile, and reliable two-step coupling procedure for molecules A and B, widely adopted in biosciences, drug discovery, and materials science.
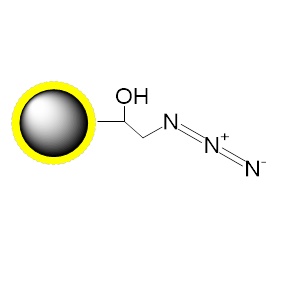
PuriMag™ G-N₃, Azide-Functionalized Magnetic Nanoparticles are uniform, polymer-coated superparamagnetic nanoparticles. Their hydrophilic surface ensures excellent dispersibility, low non-specific adsorption, and easy handling in diverse buffers. The beads feature a high-density surface coating of azide active groups, enabling covalent capture of functionalized proteins via:
-
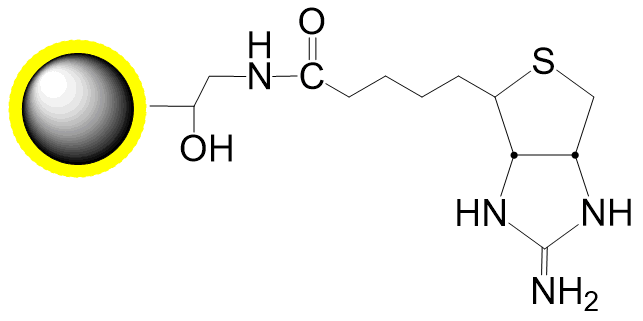
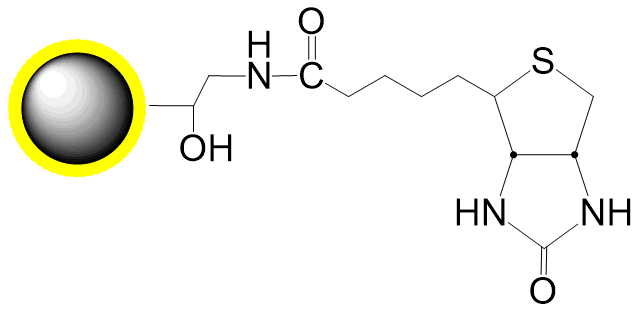
Cu(I)-catalyzed Azide-Alkyne Cycloaddition (CuAAC)
-
Cu(I)-free Azide-DBCO Click Chemistry
Workflow Requirements:
-
Target proteins must be metabolically, enzymatically, or chemically tagged with:
-
Alkyne groups (for CuAAC)
-
DBCO groups (for Cu(I)-free reactions)
-
-
Post-coupling, rigorous washing eliminates non-specifically bound proteins.
-
Unique surface chemistry ensures low background binding without surfactants.
Features & Advantages:
• High binding capacity
• Rapid, efficient coupling
• Hydrophilic long-arm spacers minimize steric hindrance/non-specific binding
• Charge-neutral surface post-coupling
• Stable covalent bonds with minimal ligand leakage
• Low non-specific binding
• Capacity: 1–20 mg protein or 0.1–2 mg peptide per gram beads
Caution: Azide beads are unstable in organic solvents and high urea concentrations.
Schematic Diagram of Bead Coupling Mechanism:

2. product description
Product Specifications
Description
Polymer coated Fe3O4 nanoparticles
Particle Size
200 nm
Number of Beads
~1.7×1010 beads/mg
Matrix
Proprietary polymer
Functional group
Azide group
Group density
~300 µmole / g of Beads
Magnetization
60~70 EMU/g
Formulation
10mg/mL in DI water
Storage
1 year at 2~8 ℃. Do not freeze.
3. Instructions for Use
A. Required Materials
tert-Butyl alcohol (t-BuOH): 12 mL
Dimethylsulfoxide (DMSO): 3 mL
Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA), M.W. 530.63: 2.7 mg
Copper(II) sulfate (CuSO₄), M.W. 159.61: 16 mg
(+)-Sodium L-ascorbate, M.W. 198.11: 20 mg
Methanol (MeOH): 4 mL
B. Coupling Method
For screening, optimize ligand immobilization density on PuriMag™ beads by testing four concentrations: 0 μM, 5 μM, 25 μM, and 125 μM.
B-1. Solution Preparation
1.Prepare 15 mL t-BuOH/DMSO (4:1) by mixing 12 mL t-BuOH + 3 mL DMSO.
2.Dissolve ligand in t-BuOH/DMSO to prepare 200 μL of 500 μM ligand solution.
3.Prepare 1 mL 5 mM TBTA (2.7 mg in t-BuOH/DMSO). Dilute 10 μL to 200 μL with t-BuOH/DMSO for 250 μM TBTA.
4.Prepare 1 mL 100 mM CuSO₄ (16 mg in H₂O). Dilute 10 μL to 200 μL with H₂O for 5 mM CuSO₄.
5.Prepare 1 mL 100 mM sodium L-ascorbate (20 mg in H₂O). Dilute 10 μL to 200 μL with H₂O for 5 mM solution.
6.Prepare 8 mL t-BuOH/DMSO/H₂O (1:1) by mixing 4 mL t-BuOH/DMSO + 4 mL H₂O.
B-2. Ligand Immobilization
1.Aliquot 2.5 mg azide beads into four tubes. Separate magnetically (RT), discard supernatant.
2.Add 500 μL t-BuOH/DMSO, disperse beads. Separate (RT), discard supernatant.
3.Repeat Step 2 twice (total 3 washes for solvent exchange).
4.Add reaction solutions in sequence (see table below). After adding 250 μM TBTA, disperse ultrasonically. Then add remaining solutions.
Concentration (um)
0
5
25
125
Azide beads (mg)
2.5
2.5
2.5
2.5
t-BuOH/DMSO (ul)
250
240
200
0
500uM ligands (ul)
0
5
25
125
250uM TBTA (ul)
0
5
25
125
Ultrapure water (ul)
250
240
200
0
5mM CuSO4 (ul)
0
5
25
125
5mM (+)-Sodium L-ascorbate (ul)
0
5
25
125
Total (ul)
500
500
500
500
5.React with tube mixer 16–20 h at RT.
6.Separate magnetically (RT), discard supernatant.
7.Add 500 μL t-BuOH/DMSO/H₂O, disperse ultrasonically.
8.Separate (RT), discard supernatant.
9.Repeat Steps 7–8 twice (total 3 washes).
10.Add 500 μL 50% MeOH, disperse ultrasonically.
11.Separate (RT), discard supernatant.
12.Repeat Steps 10–11 twice (total 3 washes).
13.Resuspend in 100 μL 50% MeOH. Store at 4°C.
(Ligand-immobilized bead concentration: 0.5 mg/20 μL)
(For research use only!)