|
Ordering information |
|||
|
Name |
Cat. No. |
Vol. |
Scheme |
|
G-Ts |
PMG011-2
|
2 ml
|
|
1. Overview
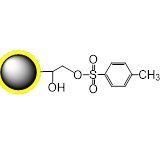
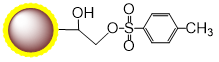
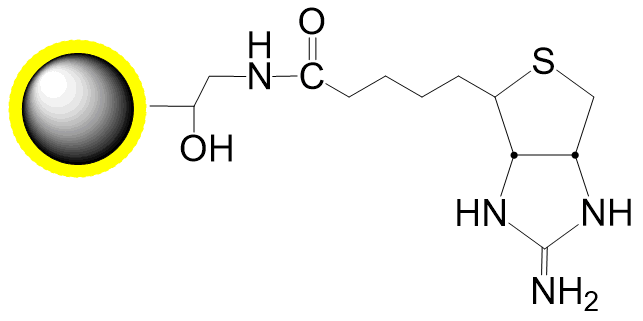
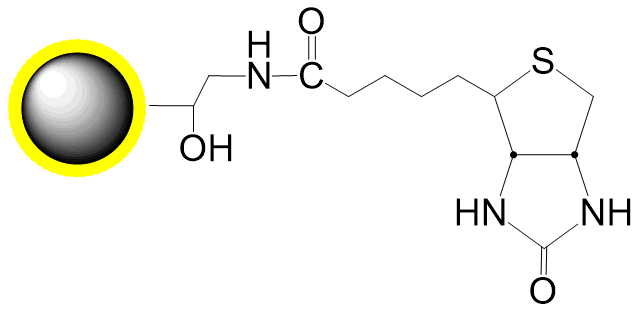
PuriMag™ G-Ts, tosyl-activated Magnetic Nanoparticles are uniform, polymer-coated superparamagnetic nanoparticles. Their hydrophilic surface ensures excellent dispersibility, low non-specific adsorption, and easy handling in diverse buffers. The beads feature a high-density surface coating of tosyl-activated groups, enabling covalent immobilization of ligands containing -NH₂ or -SH groups for bioseparation without coupling agents.
During coupling, hydrophobic tosyl groups are eliminated, converting the bead surface to hydrophilic. This chemistry preserves ligand bioactivity while achieving:
High yield in cell/bacterial lysates
Low non-specific binding
Rapid immunoassay solid-phase applications
Screening of molecular targets or phage display libraries
Ligands with affinity for specific targets (tags, receptors, enzymes) can be surface-coupled to PuriMag™ G-Ts beads.
Features & Advantages:
Pre-activated for immediate use
Optimized Coupling: Efficient at pH 7.5–9.5, 37°C
Charge-neutral surface post-coupling
Stable covalent bonds with minimal ligand leakage
Low non-specific binding
Capacity: 1–20 mg protein or 0.1–2 mg peptide per gram beads
Applications: Cell sorting, immunoprecipitation, antibody/protein/peptide/DNA/RNA purification
Schematic Diagram of PuriMag™ G-Ts Bead Coupling Mechanism:

2. product description
Product Specifications
Description
Polymer coated Fe3O4 nanoparticles
Particle Size
200 nm
Number of Beads
~1.7×1010 beads/mg
Matrix
Proprietary polymer
Functional group
Tosyl group
Group density
~300 µmole / g of Beads
Magnetization
60~70 EMU/g
Formulation
10mg/mL in 1mM HCl
Stability
pH 3.5~10, 4~80 ℃, most organic solvents
Storage
1 year at 2~8 ℃. Do not freeze.
3. Instructions for Use
Important Notes:
1.Ligands must be free of competitive agents (e.g., proteins, sugars, stabilizers).
2.Adding ammonium sulfate (Buffer C) to a final concentration of 1.0–1.5 M enhances antibody coupling efficiency.
3.Maximum covalent binding occurs after 12–18 h at 37°C. At 20°C, incubation >20 h is required. At 4°C, binding is slow (>48 h); use Buffer A for low-temperature coupling.
A. Required Materials
1.Buffers A/B/C for bead pre-wash and coupling:
Buffer A (pH-stable ligands)
Buffer B (pH-sensitive ligands)
2.Do not add proteins (except target ligands), sugars, or amines to buffers.
3.Buffers D/E for washing/storing coupled beads:
Avoid amine-containing buffers (e.g., glycine, Tris) during pre-wash/coupling.
If BSA interferes, replace with HSA or Tween 20 to reduce aggregation/non-specific binding.
For preservation, add <0.1% (w/v) NaN₃ to Buffer E.
Warning: Sodium azide reacts with copper/lead pipes to form highly explosive metal azides.
4.Buffers Composition
Buffer A (0.1 M borate buffer pH 9.5): 6.18 g H3BO3 (MW 61.83). Dissolve in 800 mL distilled water. Adjust pH to9.5 using 5M NaOH and adjust volume to 1 L with distilled water.
Buffer B (0.1 M Na-phosphate buffer, pH 7.4): 2.62 g NaH2PO4 × H2O (MW 137.99) and 14.42 g Na2HPO4 × 2 H2O (MW177.99). Adjust volume to 1 L with distilled water.
Buffer C (3 M ammonium sulphate in Buffer A or B): 39.64 g (NH4)2SO4 dissolved in Buffer A or B. Adjust pH with NaOH or HCl. Adjust up to 100 mL with Buffer A or B.
Buffer D (PBS pH 7.4 with 0.5% (w/v) BSA): Add 0.88 g NaCl (MW 58.4) and 0.5% (w/v) BSA to 80 mL 0.01 M sodium phosphate pH 7.4. Mix thoroughly and adjust volume to 100 mL with 0.01 M sodium-phosphate pH 7.4.
Buffer E (PBS pH 7.4 with 0.1% (w/v) BSA): Add 0.88 g NaCl (MW 58.4) and 0.1% (w/v) BSA to 80 mL 0.01 M sodium phosphate pH 7.4. Mix thoroughly and adjust volume to 100 mL with 0.01 M sodium-phosphate pH 7.4.
B. Coupling Protocol
B-1. Bead Washing
1.Resuspend PuriMag™ beads in original vial (vortex >30 s or tilt-rotate 5 min).
2.Transfer required bead volume to centrifuge tube.
3.Add equal volume of Buffer A or B (minimum 1 mL). Resuspend.
4.Place tube on magnetic stand. Discard supernatant.
5.Remove tube from stand. Resuspend beads in equal volume Buffer A/B (matching initial bead volume).
B-2. Ligand Coupling to Beads
*Scale for >10 mg beads. Standard: 2.5 mg beads (~250 μL), 50 μg ligand/mg beads. Optimal concentration: 2.5 mg/mL.*
1.Transfer 250 μL washed beads to new tube. Place on magnetic stand. Discard supernatant.
2.Resuspend beads in 100 μg ligand + Buffer A/B to 150 μL total volume. Mix by vortex/pipetting.
3.Add 100 μL Buffer C. Mix by pipetting.
4.Incubate with rotation at 37°C for 12–18 h.
5.Place tube on magnetic stand. Discard supernatant.
6.Add 1 mL Buffer D. Incubate at 37°C for 1 h with agitation.
7.Place on magnetic stand. Discard supernatant.
8.Add 1 mL Buffer E. Vortex 5–10 sec.
9.Place on magnetic stand. Discard supernatant.
10.Repeat Steps 8–9.
11.Resuspend in Buffer E to desired concentration.
B-3. Target Protein Isolation
Typical binding: 1–20 μg target protein per mg coupled beads (optimize per application).
1.Add 1–20 μg target protein sample to 100 μL ligand-coupled beads.
2.Tilt-incubate to capture target.
3.Place tube on magnetic stand for ≥2 min (longer for viscous samples). Discard supernatant.
4.Add 1 mL Buffer D or E. Vortex 5–10 sec.
5.Place on magnetic stand. Discard supernatant.
6.Repeat Steps 4–5 twice.
(For research use only!)