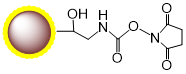Coupling Protocol C: Single-Step Coupling
(Fast protocol where beads, EDC, and ligand are added simultaneously. Best for oligonucleotides/small molecules when activation doesn't interfere)
-
Ensure protein/ligand is in amine-free coupling buffer. Use 50–400 μg protein or 1–5 nmol oligonucleotide per 100 μL coupling buffer. Keep on ice.
-
Vortex carboxyl beads to resuspend. Transfer 100 μL beads to a 1.5 mL tube. Separate magnetically and remove supernatant.
-
Add 200 μL coupling buffer, vortex vigorously for 20 sec. Separate magnetically and remove supernatant.
-
Repeat wash (Step 3) twice more.
-
Remove tube from magnetic stand. Add 50–400 μg ligand in 80 μL coupling buffer to the beads.
-
Incubate at RT for 30 min.
-
Prepare fresh EDC solution (50 mg/mL) in coupling buffer.
-
Add 20 μL fresh EDC solution to the bead mixture. Vortex to mix. Incubate at RT for 0.5–4 h with continuous mixing.
-
Separate magnetically and remove supernatant (Save for analysis if optimizing).
-
Add 500 μL quenching buffer to beads. Vortex for 20 sec. Separate magnetically and remove supernatant.
-
Add 500 μL quenching buffer to beads. Incubate at RT for 30–60 min. Separate magnetically and remove supernatant.
-
Add 500 μL quenching buffer to beads. Vortex vigorously for 20 sec. Separate magnetically and remove supernatant. Repeat this wash twice more.
-
Remove tube from magnetic stand. Add 100 μL storage buffer. Vortex to mix and store beads at 2–8°C.
Appendix: Immobilization of Small Molecule Compounds Containing Primary Amines onto Carboxyl Magnetic Beads in Organic Phase
For screening purposes, it is essential to first optimize the amount of ligand immobilized on the beads. The immobilized ligand density can be varied by altering ligand concentration. This protocol demonstrates a method for immobilizing ligand concentrations in four steps (0 mM, 0.1 mM, 0.3 mM, and 1 mM) onto COOH-functionalized magnetic beads.
1. Materials
1.1 Beads & Ligand
・ PuriMag™ G-COOH Carboxyl Beads: 10 mg (Functional group density: Approx. 200 nmol/mg)
・ Ligand: ~2 mg
1.2 Reagents
・ N,N’-Dimethylformamide (DMF): 18 mL
・ N-Hydroxysuccinimide (NHS), M.W. 115.09: 0.1 g
・ 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC・HCl), M.W. 191.70: 100 mg
・ Aminoethanol, M.W. 61.08: 200 μL
・ Triethylamine, M.W. 101.19
・ Methanol (MeOH): 4 mL
1.3 Equipment
Centrifuge, Vortex Mixer, Rotating Mixer, Ultrasonic Disperser, Magnetic Stand
2. Method
2.1 Mechanism
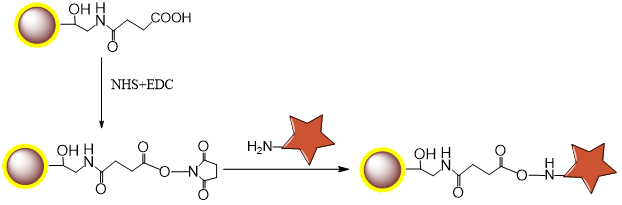
2.2 Procedure
2.2.1 Active Esterification
-
Prepare 500 μL of 1M NHS solution by dissolving NHS in DMF.
-
Transfer carboxyl beads into a 1.5 mL centrifuge tube. Repeat for another tube (total 10 mg beads).
-
Perform magnetic separation at RT. Discard supernatant.
-
Add 500 μL DMF, disperse beads.
-
Perform magnetic separation at RT. Discard supernatant.
-
Repeat steps 4-5 twice more (total 3 washes).
-
During washing, add 38.4 mg (200 μmol) EDC・HCl to a new 1.5 mL tube.
-
After DMF washes, add 800 μL DMF to beads and disperse.
-
Add 200 μL prepared 1M NHS solution, mix well.
COOH beads (mg)
5
DMF (μl)
800
1M succinimide (μl)
200
EDC.HCl (mg)
38.4
Total (μl)
1000
-
Transfer entire volume from step 9 into the tube from step 7. Mix well.
-
React at RT for 2 h with vortex mixing.
-
Perform magnetic separation at RT. Discard supernatant.
-
Add 500 μL DMF, disperse beads.
-
Perform magnetic separation at RT. Discard supernatant.
-
Repeat steps 13-14 four more times (total 5 washes).
-
Disperse beads in 100 μL DMF each (NHS-activated bead concentration: 2.5 mg/50 μL).
2.2.2 Ligand Immobilization
-
Prepare 1 mL of 1 mM ligand solution by dissolving ligand in DMF.
-
Prepare 3 mL of 1M aminoethanol solution in DMF.
-
Aliquot 2.5 mg (50 μL) NHS-activated beads into four microtubes. Perform magnetic separation at RT. Discard supernatant.
-
Add 200 μL DMF to each tube, disperse beads. Perform magnetic separation at RT. Discard supernatant.
-
Add DMF and prepared ligand solution. Disperse beads via ultrasonication.
Concentration (mM)
0
0.1
0.3
1
NHS beads (mg)
2.5
2.5
2.5
2.5
DMF (μl)
500
450
350
0
1mM ligand (μl)
0
50
150
500
Total (μl)
500
500
500
500
Note 1: To prevent localized high concentration, add ligand solution after DMF has been added to beads.
Note 2: For hydrochloride salt ligands, add two molar equivalents of triethylamine. Prepare 4 mM triethylamine and 2 mM ligand solutions, then mix equal volumes. -
React at RT for 70 min with vortex mixing.
-
Perform magnetic separation at RT. Transfer supernatant to new tube (Supernatant A).
-
Add 500 μL aminoethanol solution to remaining beads. Disperse via ultrasonication.
-
React at RT for 2 h with vortex mixing (blocks unreacted carboxyl groups).
-
Perform magnetic separation at RT. Transfer supernatant to new tube (Supernatant B).
-
Add 500 μL 50% MeOH, disperse beads via ultrasonication.
-
Perform magnetic separation at RT. Discard supernatant.
-
Repeat steps 11-12 twice more (total 3 washes).
-
Disperse beads in 100 μL 50% MeOH and store at 4°C (ligand-immobilized bead concentration: 0.5 mg/20 μL).