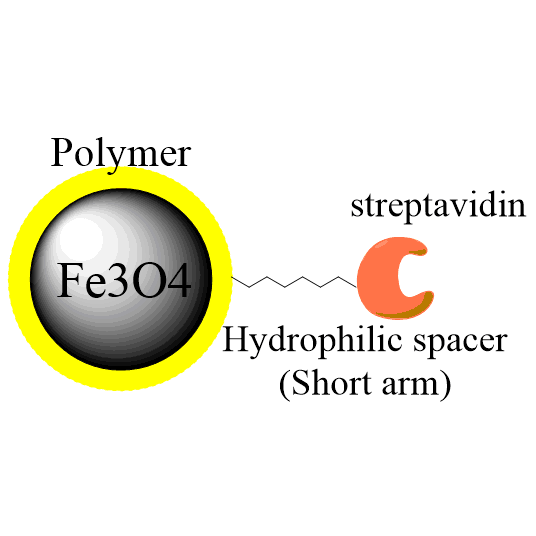
Coupling Protocol B: Two-Step Coupling Supplemented with Sulfo-NHS
(Similar to Protocol A but uses Sulfo-NHS to stabilize intermediates and enhance efficiency)
-
Ensure protein/ligand is in amine-free coupling buffer. Use 50–400 μg protein per 100 μL coupling buffer. Keep on ice.
-
Vortex carboxyl beads to resuspend. Transfer 100 μL beads to a 1.5 mL tube. Separate magnetically and remove supernatant.
-
Add 200 μL coupling buffer, vortex vigorously for 20 sec. Separate magnetically and remove supernatant.
-
Repeat wash (Step 3) twice more.
-
Prepare fresh solutions in coupling buffer: EDC (50 mg/mL) and Sulfo-NHS (50 mg/mL).
-
Add 60 μL coupling buffer, 20 μL fresh EDC solution, and 20 μL fresh Sulfo-NHS solution to the beads.
-
Mix and incubate at RT for 15 min. Separate magnetically and remove supernatant.
-
Wash PuriMag™ beads with 200 μL coupling buffer. Vortex to mix. Separate magnetically and remove supernatant.
-
Add 100 μL coupling buffer and 50–400 μg protein/ligand. Vortex to mix. Incubate at RT for 0.5–4 h.
-
Place tube in magnetic stand, separate magnetically, and remove supernatant (Save for analysis if optimizing).
-
Add 250 μL quenching buffer to beads. Vortex for 20 sec. Separate magnetically and remove supernatant.
-
Add 500 μL quenching buffer to beads. Incubate at RT for 30–60 min. Separate magnetically and remove supernatant.
-
Add 250 μL quenching buffer to beads. Vortex vigorously for 20 sec. Separate magnetically and remove supernatant.
-
Remove tube from magnetic stand. Add 100 μL storage buffer. Vortex to mix and store beads at 2–8°C.