磁性纳米颗粒:合成、表面修饰及其在药物传递中的应用
1. 介绍
磁性纳米颗粒(MNP)由于其生物相容性、易于表面修饰和磁性等特性,在生物医学和工业应用中得到了广泛的关注。磁性纳米颗粒可以以多种方式使用,与一般的纳米颗粒非常相似。然而,这些粒子的磁性增加了一个新的维度,在外部磁场的作用下,它们可以被操纵。这一特性开辟了新的应用,将药物附着在磁性颗粒上,利用磁场在体内靶向。通常,靶向是通过附着一个分子来识别另一个特定于期望目标区域的分子来实现的。这通常需要一种化学识别机制,但并不像设计的那样成功。因此,磁性纳米颗粒可以提供一种将药物运送到人体所需部位的解决方案。磁性纳米颗粒虽然可能含有其他元素,但通常是氧化铁。最常见的氧化铁有磁铁矿(Fe3O4)、磁赤铁矿(γ-Fe2O3)、赤铁矿(α-Fe2O3)和地长石。根据实验条件的不同,可以形成一种或多种氧化铁相。仔细控制实验条件以确保单相的存在是非常重要的。
在应用中经常遇到的氧化铁纳米颗粒是超顺磁性的。超顺磁性是磁性的一种形式,可以用小的铁磁性或铁磁性纳米颗粒观察到。在足够小的纳米颗粒中,磁化可以在温度的影响下随机翻转纳米颗粒的方向。而超顺磁性纳米粒子的磁化率要比顺磁性纳米粒子大得多。超顺磁性发生在具有单一磁畴的纳米颗粒中,即由单一磁畴组成。在这种情况下,可以认为纳米粒子的磁化是一个单巨磁矩,即纳米粒子原子所携带的所有单个磁矩的总和。当外部磁场作用于超顺磁性纳米粒子时,它们倾向于沿着磁场排列,导致净磁化。然而,在没有外部磁场的情况下,偶极子是随机定向的,并且没有净磁化。最近研究了由多元醇非水均相溶液合成的Fe3O4纳米颗粒磁性能的大小依赖性(Caruntu等人,2007)。在前面提到的氧化铁中,磁铁矿和磁铁矿是超顺磁性的,这些研究将主要集中在作为磁性纳米颗粒的研究中。
本文综述了近年来对磁性纳米颗粒的毒性研究,以说明磁性纳米颗粒的生物相容性。利用MNPs进行靶向药物的研究是非常有前途的,并给出了一些实例。热疗是一种利用磁场提高温度并导致细胞死亡的肿瘤的补充治疗方法,可以使用MNPs实现,并介绍了该领域的一些最新进展。最后,表格总结了用于药物输送应用的不同类型的MNP矩阵。
2. 毒性
磁性纳米颗粒在生物医学应用中引起兴趣的主要原因之一是它们的生物相容性。由于这些颗粒被用作药物传递载体,因此应详细研究它们的细胞毒性。在体外和体内的一些研究表明,这些颗粒对人体具有低毒性。铁通常通过转铁蛋白运输,它可以结合细胞表面定位的转铁蛋白受体。在细胞质中,大部分的细胞质铁储存在一种叫做铁蛋白的特殊蛋白质中。由于铁的生理相关性,MNPs最初被认为是无细胞毒性的。MNPs可以被自然分解,从而释放出三铁,然后参与正常的铁代谢。然而,人们已经认识到,MNPs的小尺寸可能会造成额外的危害,因为这些颗粒可以在细胞内达到很高的局部浓度,并且通常更难以有效地从体内清除(Rivera等人,2010;Chan et al., 2002)。此外,游离铁与自由基的形成有关,自由基对已经被病理过程削弱的神经组织尤其有害(Winer et al., 2011)。
值得注意的是,在几乎所有的研究中,毒性都显示在一定剂量以上显著增加。尽管MNPs的高负荷(100 μg/mL)会引起细胞毒性,但药物递送应用所需的浓度通常低于适当包被的MNPs的毒性水平(Karlsson等人,2008)。
毒性通常是血清蛋白与MNPs表面结合的结果,改变了细胞所接触的细胞培养基的组成(Mahmoudi等,2009)。包被的纳米颗粒毒性较低,这不仅是因为包被具有生物相容性,还因为介质中蛋白质、离子和其他成分的吸附位点较低(Mahmoudi et al., 2010)。
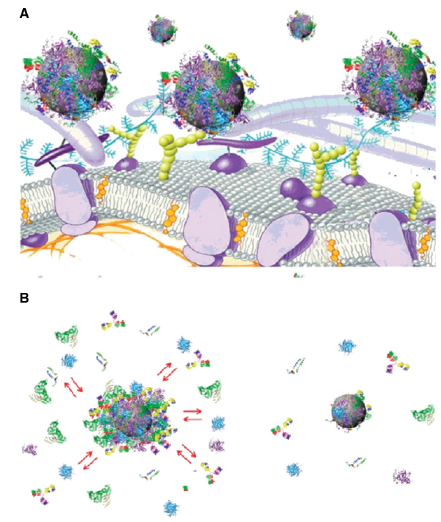
Figure 1. A) Schematic representation of the possible exchange/interaction scenarios at the bionanointerface at the cellular level. (B) Schematic drawing of the structure of protein–nanoparticle in blood plasma confirming the existence of various protein binding (e.g. an outerweakly interacting layer of protein (full red arrows) and a hard slowly exchanging corona of proteins (right) (Mahmoudi et al., 2011)
磁铁矿(Fe3O4)和磁铁矿(γ-Fe2O3)由于具有氧化/还原反应的能力而表现出不同的细胞反应。事实上,与磁铁矿相比,在没有降低细胞活力的情况下,在A549人肺上皮细胞系中,磁铁矿已被证明会导致更高水平的氧化性DNA损伤(使用彗星测定法),因为磁铁矿具有氧化的潜力(Karlsson等人,2009;Karlsson et al., 2008)。
其中一个最敏感的毒性参数是纳米颗粒的表面涂层。表面覆盖的程度被认为是细胞摄取的主要参数,因为不完全的表面覆盖被证明可以促进调理作用和快速内吞作用,而完全包裹的MNPs可以逃脱调理作用,从而延长血浆半衰期(Jung et al., 1995)。带负电荷的未包被MNPs已被证明在一定阈值以上表现出细胞毒性。在体内研究中,未包被的MNPs也具有低溶解度,这导致它们在水介质中沉淀,阻碍血管。为了降低MNPs的毒性,使用了不同的涂层。Häfeli等人(Häfeli等人,2009)用聚氧化物(PEO)三嵌段共聚物(PEO- cooh -PEO)涂覆MNPs,发现PEO尾部嵌段长度与毒性呈负相关。PEO尾巴长度在2 kDa以上,适合在体内应用。Mahmoudi等人(Mahmoudi等人,2009)表明,未包覆的颗粒比包覆聚乙烯醇(PVA)的磁铁矿颗粒具有更大的毒性。他们还表明,用表面饱和的未包覆颗粒取代,可以显著降低未包覆颗粒的毒性。用二巯基琥珀酸(DMSA)包覆磁铁矿颗粒几乎可以消除这些颗粒的毒性(Auffan et al., 2006),防止颗粒与人皮肤成纤维细胞直接接触。然而,在另一项使用DMSA包被的磁铁矿颗粒的研究中,开发了一个可量化的细胞系统模型,并表明即使是中等水平的MNPs在细胞间传递也可能对细胞功能产生不利影响(Pisanic et al., 2007)。将磁铁矿颗粒涂覆聚乙烯亚胺(PEI)-聚乙二醇(PEG),并将其毒性与支化PEI涂层进行比较(Schweiger et al., 2011)。引入聚乙二醇被证明具有屏蔽作用,并导致较低的毒性。Lee等人(Lee等人,2011)使用乙二醇双层稳定的磁铁矿纳米颗粒,并证明这些纳米颗粒无毒。磁铁矿颗粒的聚乙二醇涂层也被证明可以降低毒性(Zhou et al., 2011)。
当MNPs被嵌入壳聚糖中以获得磁性壳聚糖颗粒时,由于MNPs被壳聚糖完全覆盖,它们表现出相对较低的细胞毒性(Park等,2005)。
虽然葡聚糖是一种复杂的支链葡萄糖,经常用于医疗应用,但葡聚糖包被的磁铁矿颗粒与未包被的磁铁矿颗粒造成的细胞死亡一样多(Berry et al., 2003)。相反,在一项比较研究中,研究了未包被的磁铁矿、未包被的磁铁矿、葡聚糖包被的磁铁矿和葡聚糖包被的磁铁矿的细胞毒性,两种样品的细胞毒性均未低于100 mg/mL,唯一显示遗传毒性的样品是葡聚糖包被的磁铁矿(Singh et al., 2012)。在更广泛的研究中,Ding et al. (Ding et al., 2010)发现右旋糖酐杂交磁铁矿纳米颗粒的细胞毒性具有细胞特异性。提示对相关细胞的毒性评价应予以关注。
磁性颗粒周围的一系列次级表面活性剂已经在体内进行了毒性测试。柠檬酸和褐藻酸表面活性剂的毒性明显低于淀粉、癸酸和聚乙二醇。这项研究显示了优化表面涂层以最小化毒性的重要性(Kuznetsov et al., 1999)。
在一项体内研究中,白蛋白包被的磁铁矿微球被证明具有良好的耐受性(Kuznetsov等人,1999年)。与单剂量的阿霉素相比,含有阿霉素(一种抗癌药物)的磁铁矿白蛋白微球对动物器官或细胞的毒性降低,显著减少了副作用(Ma等人,2000年)。然而,在另一项研究中,白蛋白衍生的MNPs被发现会导致膜破坏,可能是由于蛋白质与膜脂肪酸和磷脂的相互作用(Berry et al., 2003)。
在细胞水平上评估了Fe3O4-PLLA-PEG-PLLA(聚l -乳酸)颗粒与未包覆磁铁矿颗粒相比的低细胞毒性。它们还在分子水平上产生低基因毒性和免疫毒性。急性毒性试验显示其毒性相当低,因此在生物医学应用方面具有很大的潜力(Chen等人,2012年)。包裹在MPEG-PLGA (PLGA:聚乳酸-羟基乙酸)胶束中的磁铁矿没有细胞毒性(Ding et al., 2012)。
最近,粒径而非包覆度被认为是影响巨噬细胞摄取速率的主要因素(Raynal et al., 2004)。在体内研究中,使用了50 nm(葡聚糖包覆)和4 μm(聚苯乙烯包覆)的MNPs,并证明其在眼内应用是安全的(Raju等,2011)。口服、静脉注射和腹腔注射约20 nm的MNPs没有表现出毒性(zeng et al., 2005)。含有1,6己二胺的MNPs经大鼠脑内或动脉内接种后显示是安全的(Muldoon等,2005)。40 nm的MNPs对mES细胞无毒(Shundo et al., 2012)。
在磁场作用下,MNPs显示出更高的毒性,导致细胞死亡(Simioni et al., 2007;Bae et al., 2011)。这是肿瘤治疗的基础,热疗,这将在后面的章节中详细总结。
尽管MNPs是常规使用,但长期影响和潜在的神经毒性尚未得到广泛评估(Yildirimer et al., 2011)。
磁性纳米颗粒具有较低的细胞毒性,因此在生物医学领域的应用引起了科学界对将其用作药物载体的极大兴趣。在给药方面,主要有两个目标;首先是将药物靶向到体内所需的区域,以减少对其他器官的副作用;其次是药物的控制释放,以避免经典的过量/不足周期。磁性纳米颗粒可能为这两个目标提供解决方案。磁性纳米颗粒周围的涂层经过优化,可以像大多数纳米颗粒一样以所需的方式携带和释放药物。然而,这些粒子的独特性质是它们是磁性的,允许使用外部磁场来操纵。这形成了磁性靶向的基础,其中携带药物的磁性颗粒在施加磁场时被定向到特定区域。
3.磁定位
为了在体外研究磁靶向,建立了一个模拟肿瘤供血分支动脉的实验装置,使其参数接近真实系统。实现了粒子的靶向,并发现其依赖于分支点的磁性体积力(Gitter et al., 2011)。利用相同的设置,构建了结合特征点磁体积力、磁体位置和定量数据的新型定量定位图。高达97%的纳米颗粒被成功地靶向到所选的分支中(Gitter et al., 2011)。研制了一种磁性靶向给药系统(MT-DDS)装置,该装置通过控制超导磁体产生的磁场强度和/或梯度,实现了药物在体内局部病变部位的导航和积累。将Mn-Zn铁氧体颗粒注入实验装置中,形成y形玻璃管的静脉模型。这是磁靶向药物输送系统的一项基本技术,可在循环器官系统的血管中提供药物导航,这显示了MT-DDS药物输送方法的实用性(Mishima et al., 2007)。
为了验证MNP在血管中的植入,并将注射的MNP靶向到这些特定的部位,我们建立了实验和计算模型。为了产生强大的局部场梯度,使用改进的软光刻技术构建了嵌入磁锚的微流体通道来分析捕获过程。实验调查的定性结果证实了该方法的合法性。研究表明,在血管系统的特定点捕获和聚集磁性微球是可能的(Forbes et al., 2003)。
理论模拟和实验证明了利用两种磁源局部靶向给药是一种优化高浓度磁性载体递送到人体特定部位的新方法。实验结果表明,在与人体冠状动脉的尺寸和流速相一致的流动条件下,在相当高的浓度下捕获直径为微米和亚微米的超顺磁珠是可能的。同样的实验用非磁性网进行,结果没有明显的捕获,这表明植入物负责提供必要的磁场梯度和力来捕获注射的微珠(Yellen et al., 2005)。
磁性靶向的体内研究有几项。磁性壳聚糖纳米颗粒成功靶向肿瘤组织进行光动力治疗,导致其在皮肤和肝脏组织中的低积累(Sun et al., 2009)。
磁性碳纳米管(MNT)的内表面有一层磁铁矿纳米颗粒,其中化疗药物掺入孔中。通过使用外部放置的磁铁将药物基质引导到局部淋巴结,MNTs可以在引流的靶向淋巴结中保留数天并持续释放化疗药物(Yang et al., 2008)。
在一项体外研究中,含有抗癌药物的磁性聚(乙基-2-氰基丙烯酸酯)(PECA)纳米颗粒被证明在外磁场下释放药物并具有磁性迁移率(Yang et al., 2006)。
与静脉注射相比,在颈动脉内注射聚乙烯亚胺(PEI)修饰的磁性纳米颗粒并结合磁性靶向治疗可使颗粒的肿瘤夹闭率增加30倍(Chertok等人,2010)。
采用磁铁矿-葡聚糖复合颗粒给药米托蒽醌。磁性靶向治疗后,肿瘤组织中的米托蒽醌浓度始终显著升高,磁铁应用后血浆铁浓度下降,表明磁性靶向治疗的有效性(Krukemeyer et al., 2012)。
在另一项研究中,米托蒽醌与超顺磁性fe3o4纳米颗粒结合,载药纳米颗粒通过靠近肿瘤的股动脉给药。在应用过程中,磁性纳米颗粒被聚焦的外部磁场吸引到肿瘤上。高效液相色谱-生物分布实验的结果表明,与常用的全身应用相比,磁性药物靶向可以使治疗剂在所需的身体隔室(即肿瘤区域)的富集程度提高50倍(Alexiou等,2011)。
在体外研究了由磁铁矿羧基改性聚二乙烯基苯和含磁铁矿组成的磁性纳米粒子种子在植入体辅助磁性药物靶向(IA-MDT)中的应用。在70mT的外部磁场下,首先从通过70%多孔聚合物支架的流体中捕获MNP种子,该支架设计用于模拟毛细血管组织。然后将其用于捕获具有相同磁场的磁性药物载体颗粒(MDCPs) (Mangual等,2011)。
聚[苯胺-co- n -(1- 1-丁酸)苯胺](SPAnH)用1,3-双(2-氯乙基)-1-亚硝基脲(BCNU)包覆Fe3O4颗粒。结合- bccu -3可以在体外和体内通过外部施加的磁铁集中在目标部位。当应用于脑肿瘤时,发现磁靶向可以增加结合的bccnu -3的浓度和保留率(Hua et al., 2011)。
磁性靶向增强了胶质肉瘤中淀粉包被的超顺磁性纳米颗粒的积累,并通过磁共振成像进行了量化(Chertok等人,2008)。
聚乙二醇改性交联淀粉包覆磁铁矿颗粒的体内磁靶向研究。在9l -胶质瘤大鼠模型中证实了静脉注射PEG-MNPs的选择性,增强脑肿瘤靶向性。肿瘤靶向结果是很有希望的,并为进一步开发载药PEG-MNPs和同时优化所使用的磁靶向策略提供了依据(Cole et al., 2011)。
制备了由磁性Fe3O4 (SHMNPs)核和水稳定自掺杂聚[N-(1-一丁酸))苯胺(SPAnH)壳组成的超高磁化纳米载体(SHMNCs),具有高阿霉素(DOX)载药量(27.1% wt%)。这些纳米载体增强了药物的热稳定性,并最大限度地提高了通过磁靶向治疗给药MGH-U1膀胱癌细胞的效率,部分原因是避免了p糖蛋白(P-gp)泵提高细胞内DOX浓度的作用(Hua等,2011)。
磁性颗粒也可以瞄准肿瘤区域,因此可以对肿瘤进行成像。氧化铁颗粒常被用作MRI造影剂。事实上,磁铁矿是FDA批准的造影剂。由于本研究的重点是对磁性给药技术做一个全面的总结,因此本文将不涉及用于MRI的磁性颗粒。
从上述研究中可以看出,磁靶向是一种将药物靶向到所需区域(通常是肿瘤)的有效方法。然而,在一些研究中,与磁靶向同时使用的还有靶向配体。在没有磁性靶向的情况下,靶向是利用药物载体上的配体特异性结合靶区域的受体来实现的。用于此目的的常见配体是叶酸(或叶酸)。叶酸对叶酸受体蛋白有很高的亲和力,叶酸受体蛋白通常在许多人类癌症的表面表达。如果将叶酸标记在携带药物的纳米颗粒上,叶酸就会与癌细胞表面的叶酸受体结合,并通过内吞作用摄取缀合物,完成靶向药物递送。磁性粒子与靶配体的示意图如图2所示。
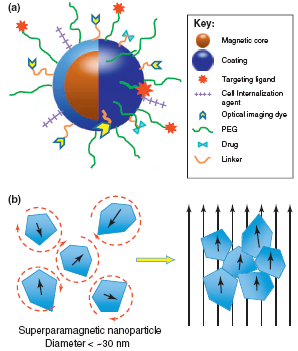
Figure 2. a) Schematic representation of the ‘‘core–shell’’ structure of MNPs and multi-functional surface decoration. MNPs consist of a magnetic iron oxide core coated with a biocompatible material (e.g. polysaccharide, lipid, protein, small silane linkers, etc.). Functional groups on the surface of coatings are often used to link ligands for molecular targeting, cellular internalization, optical imaging, enhanced plasma residence and/or therapy. The variety of moieties that decorate the MNP surface imparts the nanoparticle with its multi-functional, theranostic character. (b) Illustration of superparamagnetic MNP response to applied magnetic fields. MNPs comprise rotating crystals that align with the direction of an applied magnetic field. Crystal reorientation provides the high magnetic susceptibility and saturation magnetization observed for this material. The circular dashed lines around the superparamagnetic nanoparticles on the left illustrate the randomization of their orientation, due to temperature effects, in the absence of a magnetic field. (Cole et al.2011).
以生物相容性Pluronic F127和与叶酸(FA)化学偶联的聚乳酸(F127- pla)共聚物为载体,以超顺磁性氧化铁颗粒为基础合成磁性纳米载体。磁颗粒在外加磁场的作用下被引导到靶部位,从而提高抗肿瘤药物的治疗效果。这些定性结果都是通过简单的统计分析进行的,这说明双重靶向机制可以带来更好的治疗效果(Huang et al., 2012)。
超顺磁性氧化铁纳米晶体和DOX与胆固醇(含或不含叶酸)共封装到PLGA/聚合脂质体核壳纳米载体中。在体外实验中,叶酸靶向DOX负载磁性核壳纳米载体对Hela细胞的靶向效果优于非叶酸靶向载体(Wang et al., 2012)。
热敏磁性脂质体具有DPPC:胆固醇:DSPE-PEG2000:DSPE-PEG2000-叶酸(DPPC:双棕榈酰磷脂酰胆碱;DSPE: 1,2-二硬脂酰-sn-甘油-3-磷酸乙醇胺),摩尔比为80:20:45:0.5。与Caelyx®(一种市售的多柔比星脂质体制剂)、非磁性叶酸靶向脂质体(FolDox)和表达叶酸受体的肿瘤细胞系(KB和HeLa细胞)中的游离DOX相比,该载体通过永久磁场物理靶向肿瘤细胞时,DOX的细胞摄取显著增加(Pradhan等,2010)。
制备了具有介孔二氧化硅核壳结构的磁性纳米颗粒,并成功地以荧光聚合物链作为标记段,叶酸作为癌症靶向段,装载药物进行定向释放。药物载体被证明能够钻入细胞膜并获得抗癌药物持续释放到细胞质中。体外细胞对药物的摄取表明,载药纳米复合材料可以有效靶向肿瘤细胞(Chen et al., 2010)。
合成了具有荧光SiO2外壳的Fe3O4纳米颗粒,并与叶酸共轭的超支化聚甘油(聚丙烯酰胺接枝Fe3O4@SiO2纳米颗粒)接枝。体外研究表明,与巨噬细胞和成纤维细胞相比,人卵巢癌细胞(SKOV-3)对叶酸结合纳米颗粒的摄取明显优先(Wang等,2011)。
磁铁矿纳米颗粒通过吸附微粒表面的聚合层(羧甲基壳聚糖)进行修饰,并与荧光染料、靶向配体和药物分子结合,以改善靶向特异性诊断和可能的治疗应用。将丙烯酸、叶酸、颗粒(Fe3O4-CMC-AA-FA)和DOX加载到MNPs的外壳中,并在不同ph下进行释放研究。与NIH3T3细胞相比,Fe3O4-CMC-AA-FA-DOX NPs对HeLa细胞的生长有明显的抑制作用,且呈剂量依赖性。该研究表明,Fe3O4-CMC-AA-FA能够提供单一纳米级结构,具有肿瘤细胞靶向、成像和药物递送功能。这是首次描述基于壳聚糖的MNPs系统具有上述所有功能(Sahu et al., 2012)。
叶酸以外的其他配体也被用于纳米颗粒的活性靶向。DOX在5-羧基荧光素(FAM)标记的AGKGTPSLETTP肽(A54)偶联淀粉包被氧化铁纳米颗粒上证明了DOX负载的A54- ions (SION:超顺磁性氧化铁)对BEL-7402细胞的特异性。显微镜图像证明,在体内外磁场作用下,负载dox的A54-SIONs成功靶向裸鼠肿瘤组织(Yang et al., 2009)。
配体修饰CPT-SAIO@SiO2纳米载体用于递送抗癌剂(包封喜树碱(CPT))。研究发现,修饰后的纳米载体对CPT具有相当高的载药效率,并且通过网格蛋白介导的内吞作用被过表达EGFR的癌细胞摄取。通过外部磁刺激释放CPT分子在细胞内被证明在技术上是成功的,并且确保了比使用游离药物获得更高的治疗效果(Tung et al., 2011)。
西妥昔单抗-抗表皮生长因子受体(EGFR)单克隆抗体与聚乙二醇-聚(ε-己内酯)(PEG-PCL)连接的免疫胶束。这些胶束负载DOX和Fe3O4超顺磁性氧化铁。结果表明,免疫胶束比其非靶向对应物更有效地抑制细胞增殖。西妥昔单抗免疫胶束更有效地与过度表达表皮生长因子受体的癌细胞结合,导致更多的超顺磁性氧化铁和DOX被转运到这些细胞中(Liao et al., 2011)。
通过ph敏感键将抗癌药物偶联到聚乙二醇化的SPIO (SPIO:超顺磁性氧化铁)纳米载体上。肿瘤靶向配体,环(arg - gly - asp - d - ph - cys) (c(RGDfC))肽,PET 64Cu螯合剂,大环1,4,7-三氮杂环壬烷- n, N0, n00 -三乙酸(NOTA),被偶联到PEG臂的远端。体外实验表明,与不含crgd的SPIO纳米载体相比,结合crgd的SPIO纳米载体具有更高的细胞摄取水平。这些纳米载体在联合靶向抗癌药物递送和PET/MRI肿瘤双模成像方面表现出了很好的特性(Yang等,2011)。
聚脂质体(PEG/RGD-MPLs);由两亲性聚合物十八烷基季铵化改性聚γ-谷氨酸(OQPGA)、聚乙二醇化OQPGA、RGD肽接枝OQPGA和磁性纳米颗粒组成。它提供了一种对具有超顺磁特性的外部永磁体产生响应的可能性,当用于体内磁性组织靶向时。细胞摄取结果表明,PEG/RGDMPLs(含RGD和磁性颗粒)在MCF-7细胞中比非RGD和非磁性载体表现出更多的药物细胞摄取(Su et al., 2012)。
这些研究表明,磁靶向是一种有效的将药物靶向肿瘤区域的方法。结合使用合适配体的主动靶向,配体修饰的载药磁性纳米颗粒在外部磁场的作用下提供了有效的药物靶向系统。一旦将磁性颗粒靶向到所需区域,最常用的肿瘤治疗方法之一就是热疗。当磁性纳米颗粒在肿瘤附近并受到交变磁场作用时,会使肿瘤散发热量并升高肿瘤温度,导致肿瘤细胞死亡。
4. 高热治疗
温度在40°C到45°C之间通常被称为热疗。温度高达42℃可使癌细胞更容易受到辐射的影响,并导致一定程度的细胞凋亡,而温度在45℃以下被称为热消融,并导致细胞直接杀伤(坏死)(Elsherbini等人,2011)。在磁性纳米粒子热疗治疗癌症的临床应用中,在保护正常组织的同时确保对肿瘤的最大损伤是非常重要的(saloum et al., 2009)。磁性纳米粒子热疗在癌症治疗中具有很大的潜力,但由于预期的加热分布难以控制,导致肿瘤组织温度升高不均匀和不充分,严重限制了其应用。组织中颗粒的运输包括载体溶液的细胞外运输、载体溶液中颗粒的运输以及颗粒与细胞表面的相互作用等过程。纳米颗粒在肿瘤中的细胞外转运尚不清楚(saloum et al., 2008)。
热疗几乎总是与其他形式的癌症治疗一起使用,如放射治疗和化疗。热疗可能会使一些癌细胞对辐射更敏感,或伤害辐射无法伤害的其他癌细胞。当热疗和放射治疗同时进行时,它们通常在一小时内进行。热疗还可以增强某些抗癌药物的作用(Van der Zee, 2002;Wust et al., 2002)。
许多临床试验研究了热疗与放射治疗和/或化疗的结合。这些研究集中在多种癌症的治疗上,包括肉瘤、黑色素瘤、头颈癌、脑癌、肺癌、食道癌、乳腺癌、膀胱癌、直肠癌、肝癌、阑尾癌、子宫颈癌和腹膜内膜癌(间皮瘤)(Falk等人,2001;Feldman et al., 2003;Chang et al., 2001)。这些研究中的许多,但不是全部,都表明当热疗与其他治疗相结合时,肿瘤大小显著减小。然而,并非所有这些研究都表明,接受联合治疗的患者生存率提高(Van der Zee, 2002;Wust et al., 2002)。磁性纳米材料在热疗基础和联合治疗中的独特优势如图3所示。
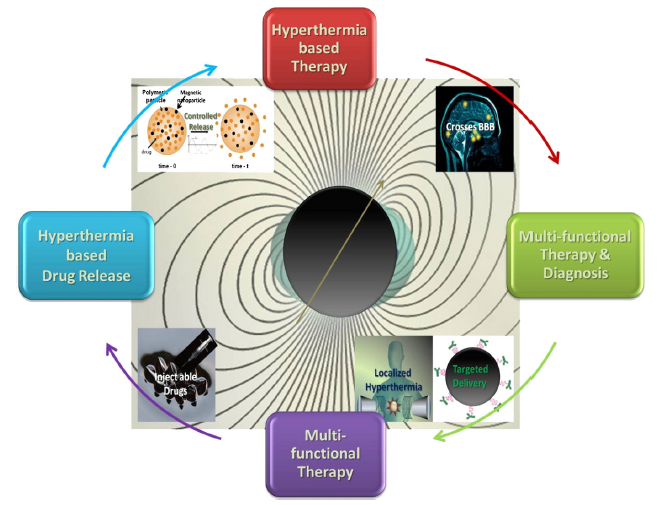
Figure 3. A schematic representation of some of the unique advantages of magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery (Kumar & Mohammad, 2011).
用于加热的交变磁场中的磁损失是由于粒子系统中磁化反转的不同过程引起的:(1)磁滞,(2)nsamel或Brown弛豫,(3)粘性悬浮液中的摩擦损失(Hergt et al., 2006)。
超顺磁性纳米粒子的磁化能在热能的作用下自发改变取向。磁化强度在两个平衡位置之间振荡。两个方向变化之间的典型时间由n 弛豫时间给出,其中τ0是一个尝试时间,其值约为10−9-10−10秒。在没有磁场的情况下,溶液中的磁性纳米颗粒会随机运动,这种运动被称为布朗运动。当磁场作用于流体中的磁性纳米颗粒时,由于磁场与磁化相互作用产生的扭矩,磁性纳米颗粒旋转并逐渐与磁场对齐。磁性纳米粒子与一个小的外部磁场对齐所花费的时间由布朗弛豫时间给出,其中η为溶剂粘度。磁场旋转和磁化旋转之间的延迟导致磁滞。磁滞回线的面积作为热能在环境中耗散,用于磁热疗。
当交变磁场(AMF)作用于磁性材料时,由于磁滞,一种称为比吸收率(SAR)的能量被耗散,并以纳米粒子的W/g表示。
给定材料的SAR由SAR = Af给出,其中a为磁滞回线面积,f为磁场交变频率。A用J/g表示,也称为材料的“比损耗”,因此在一些研究中也可以将SAR称为比损耗功率(specific Loss Power, SLP)。
几个研究小组对同一材料估计的SAR值可能会有所不同,因为它取决于几个参数,如载体流体的物理和化学性质、涂层材料、施加磁场的频率和振幅、Fe3O4纳米颗粒的大小和形状(Elsherbini等,2011)。
通过优化一种算法,可以反向确定多个纳米颗粒注入引起的最佳加热模式,从而开发出以A为单位的优化SAR分布(saloum et al., 2009)。对于热疗应用,需要高SAR值。实现这一目标的一种方法是增加磁场强度,但平均磁场强度应保持在30 mT以下,以避免形成涡流,从而诱发毒性(alphandsamry et al., 2012)。
SPA作为粒径函数的研究表明,构成加热剂的纳米颗粒的平均粒径和粒径分布是设计高效加热纳米颗粒的核心参数(Goya et al., 2008)。
不幸的是,很难对颗粒的组成和大小进行直接比较。在一项研究中,制备了多畴铁氧体颗粒,并将其与有右旋糖酐包覆和未包覆的小磁铁矿颗粒的SAR数据进行了比较。大铁氧体颗粒(200-400 nm)的每质量功率吸收比相同成分的小颗粒要低得多,尽管两种颗粒尺寸分布都相对较宽(Jordan等,1993)。然而,大尺寸的颗粒无法到达细胞内部(Martín-Saavedra et al., 2010)。
通过对Pluronic F127包覆的Fe3O4单分散颗粒进行量热测量,结果表明,在给定频率下,超顺磁颗粒的升温速率取决于颗粒大小,这与早期的理论预测一致。结果还表明,正如预测的那样,SLP随着样本多分散性而扩大(Gonzales-Weimuller et al., 2009)。
同样,单畴尺寸范围(20-70 nm)的平均粒径与小尺寸分布宽度相结合可以增强SLP (Hergt et al., 2007)。
先前的研究表明,在靶向磁热疗治疗中,组织铁浓度与加热速率之间存在线性关系(Pardoe et al., 2003)。动脉栓塞热疗(AEH)的一个关键组成部分是铁磁颗粒在正常肝实质(NHP)以及肿瘤组织中的浓度和分布。如果NHP中的颗粒分布是不均匀的,有高浓度的区域,那么在AEH期间可能会导致不需要的坏死区域(Moroz等人,2002)。
在另一项研究中,几种具有代表性的磁性氧化铁纳米颗粒用于不同的制备方法(湿化学沉淀法、研磨法、细菌合成法、磁性粒度分馏法)进行比较研究(Hergt et al., 2006)。市面上可买到的非常小的超顺磁颗粒被认为不是有效加热肿瘤的最佳材料。相反,超顺磁性磁铁矿纳米颗粒被证明适合于用射频诱导埃利希肿瘤热疗(Elsherbini et al., 2011)。
在超顺磁状态下,单个粒子的磁矩与MNPs的SPA值之间没有相关性。尽管SPA机制与初始畴壁之间的关系仍有待确定,但最佳粒径建议接近Fe3O4相从单畴到多畴转变的临界尺寸(Goya et al., 2008)。
当使用磁性纳米颗粒作为磁颗粒热疗的热源时,了解颗粒是否在组织液中自由移动或固定在肿瘤组织上是特别有趣的。固定状态决定了所给粒子的弛豫行为,从而决定了它们的比加热功率(Dutz et al., 2011)。如果粒子不能旋转,由布朗弛豫引起的温度升高可以忽略不计。有研究表明,羧甲基右旋糖酐包被的磁性颗粒注射入肿瘤后,对肿瘤组织的固定作用相当强(Dutz et al., 2011)。
研究了nsamel弛豫对无法移动或旋转的磁性纳米粒子的影响,并比较了线性和圆极化场中的损耗(De chaltel et al., 2009)。在低于拉莫尔频率的频率下,线性极化是更好的热电源,在高频率下(超过拉莫尔频率),圆极化是更好的。如果各向同性样品中的nsamel松弛是主要机制,则无需考虑在复杂几何结构中产生圆极化场的技术复杂性。
为了使组织中颗粒浓度达到所需温度,MNP的比热功率(SHP)应尽可能高。发现超顺磁粒子尺寸的比热功率与外加交变磁场的频率和振幅的关系符合弛豫理论的预测。对于较小的平均尺寸(约6纳米),加热能力小得可以忽略不计,而较大的颗粒提供适合热疗的加热(Glöckl等人,2006年)。
文献中通常报道的SLP数据显示,当场幅为10 kA m-1,频率约为400 kHz时,散射的数量级为10 - 100w g-1 (Hergt et al., 2006)。
总而言之:
为了在小肿瘤(目前直径小于10mm)中达到有效的治疗温度,MNP的SLP必须大大增加。
MPH的主要实际问题是肿瘤的MNP供应不足。对于IT注射,组织中MNP分布的不均匀性可能导致局部温度差异,从而无法区分热疗和热消融。由于肿瘤部分温度增强不足,存在存活肿瘤细胞增殖的风险。
对于MNP的全身供应(例如抗体靶向),MNP的靶富集必须大大增强以达到治疗温度。特别是,小靶点(低于目前诊断限度的转移)的治疗似乎是一个值得怀疑的希望(Hergt等人,2007)。
对热疗有用的比损失功率受到交变电场振幅和频率的严重限制。在400 kHz和10 kA m−1条件下,对于平均粒径约为18 nm的粒子,如果粒径分布足够窄,其SLP值可达数百W g−1。对于磁铁矿晶体平均直径约为35 nm的细菌磁小体,发现了接近1 kW g−1的非常大的SLP值(Hergt et al., 2006)。
生物医学中常用的氨基硅烷修饰的MNPs,细菌磁小体(BM)在AMF下表现出更好的加热效果。虽然这两种颗粒都被发现通过使用相同浓度的MNPs和磁小体热疗来增强细胞活力的降低,但脑转移瘤需要更低强度的电流才能在肿瘤细胞中产生类似的抑制作用(Liu et al., 2012)。
当磁小体链(由蛋白质组成的细丝相互结合)在癌细胞存在的情况下孵育,并暴露在频率为198 kHz、平均磁场强度为20或30 mT的交变磁场中时,它们能有效抑制癌细胞的增殖。这种行为可以解释为高细胞内化,溶液中良好的稳定性和磁小体链的均匀分布,从而实现高效加热(alphandsamry et al., 2012)。
当磁小体链被加热时,将磁小体结合在一起的细丝发生变性,得到个体磁小体,这些磁小体在交变磁场作用下容易聚集,在溶液中不稳定,不能有效抑制癌细胞增殖(alphand 等人,2012)。
聚乙二醇甲基丙烯酸甲醚和二甲基丙烯酸甲酯与氧化铁作为植入式生物材料。结果表明,可以通过改变AMF强度来控制水凝胶的温度,使凝胶达到高温(42-45℃)或热烧蚀(60-63℃)温度。水凝胶纳米复合材料达到的最终温度可以根据这些温度范围中的任何一个进行定制。水凝胶在AMF中加热,加热响应取决于凝胶中的氧化铁负载和磁场强度(Meenach et al., 2010)。
采用脂质薄膜法制备了含有磁流体和光敏剂基配合物(CB:ZnPc-ML)的阳离子磁性脂质体。这一结果表明,光和交流磁场一起应用比单独应用两种处理更有效(Bolfarini et al., 2012)。
用载药PCPG磁脂质体评价磁热疗联合化疗的效果。热敏药物释放在磁场的影响下发生,这种联合治疗被证明比单独治疗更有效(Kulshrestha et al., 2012)。
结果表明,即使吸收量相同,铁磁MNPs获得的温度也高于超顺磁MNPs获得的温度。这是由于磁滞损耗和磁松弛之间的热效率差异。热的产生主要是由于磁滞损耗而不是磁弛豫。与细胞外产生的热量相比,纳米颗粒进入细胞并吸附在细胞膜上产生的热量对细胞的破坏至关重要(Baba et al., 2012)。
根据一些人的说法,众所周知的氧化铁流体变得不受欢迎,因为它们的铁原子与血红蛋白的铁原子很难区分。一个建议的解决方案是使用混合铁氧体(MFe2O4,其中M¼Co, Mn, Ni, Zn)来具有一系列的磁性能。这些铁氧体因其节省时间、固有毒性低、易于合成、物理和化学稳定性以及合适的磁性而受到特别关注(Sharifi et al., 2012)。
Giri等人研究了100纳米以下的柠檬酸盐包覆铁氧体颗粒。发现涂层材料的饱和磁化强度降低,因为磁化强度与相同磁性材料的重量成正比。发现矫顽力足以使热疗中的迟滞损失加热。磁滞数据表明,这些样品(涂覆)表现出足够的磁滞损失,以获得破坏肿瘤细胞所需的温度。
铁素体颗粒在壳聚糖基质中以不同的比例制备(Park等人,2005)。随着壳聚糖比例的增加,达到热疗所需时间缩短,饱和磁化强度降低。优化铁素体-壳聚糖的比例在热疗方面具有广阔的应用前景。
6-12纳米的钴钛铁氧体纳米颗粒被证明适用于热疗应用(Ichiyanagia等人,2012)。Zn-Gd铁氧体颗粒是合适的,但如果你用聚乙二醇(PEG)盖住它们,它们就没有用了(Yao等人,2009)。与聚(甲基丙烯酸酯)和聚(2-羟乙基甲基丙烯酸酯)共聚的7.5 nm钴铁氧体颗粒被证明适用于热疗(Hayashi等人,2012)。CoFe2O4铁氧体颗粒(铁磁性)被证明适用于热疗(Skumiel, 2006)。
研究各种材料的纳米颗粒产生的加热,钡-铁氧体和钴-铁氧体不能产生足够的MFH加热,铁-钴产生的加热速度太快而不安全,而fcc铁-铂、磁铁矿和磁铁矿都能够产生稳定的可控加热。铁钴MNPs诱导的温度变化太大,而钡铁氧体和钴铁氧体MNPs不能提供足够的热量来治疗肿瘤。模拟表明,磁铁矿、fcc铁铂和磁铁矿MNPs非常适合MFH,可以将肿瘤加热到41°C以上,同时保持周围健康组织温度低于该值(Kappiyoor et al., 2010)。
热可逆水凝胶(波洛沙姆,壳聚糖),可容纳20% w/v的磁性微粒,被证明是不够的。然而,海藻酸盐水凝胶含有10% w/v的磁性微粒,外部凝胶化导致植入物定位到肿瘤周围,而内部凝胶化在原位失败。有机凝胶配方由溶解在单一有机溶剂中的沉淀聚合物组成,显示出不同的微观结构。在DMSO (DMSO:二甲基亚砜)中含有40% w/v磁性微粒的8%聚(乙烯-乙烯醇)形成了肿瘤铸造和热传递方面最合适的植入物(Le Renard等人,2010)。
细胞培养实验表明,通过调整磁性微球MMS的数量和AMF的暴露时间,可以将磁铁矿纳米颗粒嵌入介孔二氧化硅基质中,实现轻度到非常高强度的热处理(Martín-Saavedra et al., 2010)。对含铁的10-40纳米多壁碳纳米管的加热效果进行了研究,并证明是合适的(Krupskaya et al., 2009)。
几种磁流体被证明适合热疗应用。在一项比较研究中,对16种商业磁流体进行了调查,并区分了最合适的磁流体(Kallumadil et al., 2009)。20-30µm的磁铁矿微胶囊嵌入琼脂体中,在交变磁场下产生热量(Miyazaki et al., 2012)。气凝胶基质中10纳米的磁铁矿纳米颗粒是潜在的热疗剂,其中气凝胶基质可用于联合治疗的药物装载(Lee et al., 2012)。
从上述研究中可以看出,虽然对于理想的热疗磁性材料并没有一个明确的定义,但根据具体情况,可以采用几种材料。药物传递与热疗联合治疗在该领域具有广阔的应用前景。
磁性药物载体
由于磁性颗粒具有低毒性和靶向性,因此它们是药物递送的潜在候选者,通常将磁性颗粒涂覆以稳定其抗沉淀,确保其低细胞毒性并在基质中携带药物。
在体内应用中,SPION颗粒应被包裹以防止药物分子偶联并限制与非靶向细胞侧的相互作用,防止颗粒团聚并增强药物装载和释放。SPION涂层的不同方法导致不同的聚合物组装如图4所示。在多糖包衣和共聚物包衣中,所得到的颗粒呈均匀的包芯状。在另一种涂覆方法中,聚合物分子固定在磁性颗粒表面,形成刷状结构。脂质体和胶束形成的分子形成核壳结构,核中有磁性粒子。这些结构可用于保留疏水区域的药物包封。(Veiseh et al., 2010)。
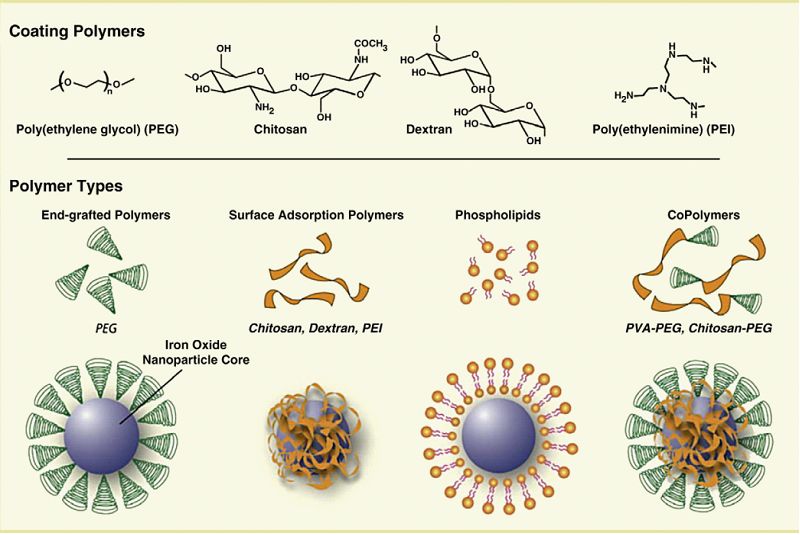
不同的颗粒被设计为药物传递载体,表1给出了这些颗粒的总结。
表1 磁性纳米材料在药物传递中的应用综述
| Type of magnetic nanoparticle | Particle size | Coating agent | Drug | Design matrix | Drug release mechanism | Ref |
| Fe3O4 |
Core diameter of 10 - 15 nm Final diameter of 160 nm |
chitosan/PAA multilayer | cefradine |
Layer-by-Layer (LBL) The drug molecules were entrapped inside the hollow spheres through diffusion process |
pH responsive | Zhang et al., 2006 |
| Fe3O4 | Final diameter of "/>1 µm | sodium carboxy methyl cellulose and chitosan | ------ | self-assembly shell composed of layers of carboxy methyl cellulose and chitosan around the magnetic core | ------------ | Cui et al., 2011 |
|
Fe3O4 |
Core diameter of 8 nm Final diameter of of 107 nm |
chitosan | cefradine |
cross-linking the particles with glutaraldehyde and the drug is embedded in the polymer matrix |
pH responsive | Li et al., 2007 |
| Fe3O4 |
Core diameter of 5 nm Final diameter of 1–1.5cm |
Alginate / chitosan | insulin | insulin encapsulation in alginate/chitosan beads. The beads containing insulin were prepared in triplicate by extrusion method. | Magnetic field | Finotelli et al., 2010 |
| Fe3O4 | Final diameter of 200 nm | multiwalled carbon nanotubes (MWNTs) | doxorubicin | The MWNT-hybrid nanocomposites provided an efficient way for the extraction and enrichment of doxorubicin via π–π stacking of DOX molecules onto the polyaromatic surface of MWNTs. | pH responsive | Shen et al., 2011 |
| Fe3O4 | Core diameter of 3 nm | CNTs | --------- | Magnetic nanoparticles adsorb on the CNT ends | ------ | Panczyk et al., 2010 |
| Fe3O4 |
Core diameter of 5–10 nm. CNTs average diameter of about 30–50 nm and average length of about 100–500 nm |
CdTe QDs and CNTs | ------- |
CNT-SPIO-CdTe nanohybrids via LBL assembly |
---------- | Chen et al., 2010 |
| γ-Fe2O3 | Core diameter of 10 nm | CNT | Diaminophenothiazine (methylene blue) | monodisperse, inherently open-ended, multi-wall CNTs loaded with magnetic iron-based nanoparticles that are encapsulated within the tube graphitic walls | Vermisoglou et al., 2011 | |
| Fe3O4 |
Core diameter of 8–12 nm mACs had a mean diameter of about 30 nm MWNTs=40-60 nm |
(mMWNTs) and magnetic-activated carbon particles (mACs) |
gemcitabine (GEM) | Fe3O4 nanoparticles are on the outer surface of the PAA functionalized MWNTs and the drug is adsorbed on the surface . | Yang et al., 2011 | |
| CoFe2O4 nanoparticles |
Core diameter of 6 nm MWCNTs with an outer diameter of 10–30 nm and an average length of 0.5–2 µm |
MWCNT/cobalt ferrite (CoFe2O4) magnetic hybrids | doxorubicin |
cobalt ferrite is on the outer surface of the MWCNT |
pH responsive | Wu et al., 2011 |
| γ-Fe203 |
Core diameter of 5 nm Final diameter of 100 nm |
DNA | fluorescein | Single-stranded DNA was immobilized onto the silica network, and the magnetic particles are loaded onto the network. The complementary DNA sequence was then attached to magnetic nanoparticles. | Temperature responsive | Ruiz-Hernandez et al., 2011 |
| Fe3O4 |
Core diameter of of 8 nm Final diameter of 150 nm |
PEG-functionalized porous silica shell | doxorubi-cin | DOZ conjugated magnetite particles are coated with silica to obtain core/shell nanoparticles and the whole composite is coated with PEG |
the breaking of the bonding of the drug to the carrier or the swelling and degradation of the polymer. |
Chen et al., 2010 |
| α-Fe2O3 |
Core diameter of 13 nm micron-sized mesoporous molecular sieves (with 2.9-nm pores) MCM-41 and MCM-48 powders gave mean pore sizes of 3.7 and 3.5 nm, a size between 1 and 4 µm. and hollow silica microcapsules (pores of 2.7, average diameter being around 3 µm. and 15 nm. 250-nm wall thickness |
hollow silica microcapsules | --------- | Magnetic particles are encapsulated inside the hollow silica microcapsules | ------------ | Arruebo et al., 2006 |
| Fe3O4 |
Core diameter of 10 nm Final diameter of 100 nm with 20 nm silica shell |
SiO2@ Fe3O4 core–shell NPs |
Silica-magnetite nanocomposites are emulsified and self-assembly of magnetic-mesoporous heteronanorods at the interface of water-in-oil droplets takes place. |
Zhang et al., 2011 | ||
| Fe3O4 |
Particles between 150nm and 4.5 µm |
silica,arabic acid and cross-linked polysaccharide |
antibody | particles with starch derivative or polymeric arabic acid as matrix material functionalized with an antibody | --- | Sieben et al., 2001 |
| Fe3O4 |
Final diameter if 202 nm |
β-cyclodextrin and pluronic polymer (F-127) | curcumin |
multi-layer polymer coating around the magnetic particle and the drug is encapsulated via diffusion into polymer matrix |
The initial burst of release was due to immediate dissociation of surface bound curcumin molecules that exist on the CD or F127 polymer matrix. The remaining sustained drug release was due to the slow release of the drug entrapped inside CD and/or F127 polymer layers. |
Yallapu et al., 2011 |
| Fe3O4 | Core diameter of 14.8 nm |
2-hydroxypropyl-cyclodextrin (HCD) onto the gum arabic modified magnetic nanoparticles (GAMNP) |
ketoprofen | polymers grafted onto magnetic particles(Multilayer polymer matrix) |
drug molecules are rapidly released from HCDGAMNP, whereas some remains associated to degredation of HCD-GAMNP |
Banerjee & Chen, 2009 |
| Fe3O4 |
Final diameter of 13 nm. |
(3-aminopropyl) triethoxysilane coated (APTES-MNPs) with b-cyclodextrin (β-CD). | ------- |
layer-by-layer |
--------- | Cao et al., 2009 |
| Fe3O4 | Core diameter of 9.2 nm | Oleic acid, sodium dodecyl benzene sulfonate SDBS, bovine serum albumin (BSA) | --- | Oleic acid capped magnetic nanoparticles are embedded in the SDBS micelle and BSA adsorbs onto the micellar entity. | ---- | Yang et al., 2009 |
| Fe3O4 | Final diameter of 300 nm |
poly (N-isopropylacrylamide) PNIPAAm and poly(D,L-lactide-co-glycolide) PLGA |
Bovine serum albumin (BSA) and curcumin |
(MLNPs) with a magnetic core and two shells made up of temperature-sensitive polymers (PNIPAAm) were encapsulated with PLGA. BSA was first loaded into PNIPAAm magnetic nanoparticles. Second, curcumin was loaded to PLGA to form the multilayer nanoparticles | Temperature responsive | Koppolu et al., 2010 |
| Fe3O4 | Final diameter of 150 nm | dextran | fluorescein (Fluo) or TEXAS RED® (Texas) fluorescent dye |
By oxidizing Ferumoxides (FE) (suspension consisting of dextran- coated SPION) hydroxyl groups on the dextran coating are oxidized to aldehyde groups. Lysine fixable fluorescein (Fluo) or TEXAS RED® (Texas) fluorescent dye (supplied as lysine fixable dextran conjugates) was reacted with aldehyde FE and the fluorescent dye is conjugated to FE SPION (FL FE). |
---- | Lee et al., 2008 |
| Fe3O4 |
Core diameter of 5 5 nm Final diameter of 4 μm, |
Fe3O4/ PAH |
fluorescein isothiocyanate (FITC)-Dextran |
layer-by-layer (LbL) assembly FITC-dextran nanoparticle is coated with PSS polyelectrolyte which contains the magnetic particles forming a magnetic shell around the particle. |
Magnetic field | Hu et al., 2008 |
| Fe3O4 | Core diameter of 12 nm | coated with starch, dextran, PEG or MPEG | ---- | Polymeric networks cover a large number of continuous magnetic monodomains. | ---- | Huong et al., 2009 |
|
magnetic fluids Carboxyde-xtran coated DDM128 P6 (dextran–magnetite) Aminosilane coated (aminosilane–magnetite) MFL AS |
DDM128 P6: core diameter of 3 nm MFL AS: core diameter of 15 nm. |
dextran- or aminosilane-coated | ---- | ----- | Jordan et al., 2006 | |
| Fe3O4 | Core diameter of 7 nm | PVA and starch | PVA coated particles as large clusters where starch coated ones are be densely dispersed in the polymeric matrix | Voit et al., 2001 | ||
| Fe3O4 | Final diameter of 110±22 nm | starch | ------ |
Core-shell particles |
----- | Chertok et al., 2008 |
|
Fe3O4 |
coated with starch (G100) particles final diameter of 110 (±22) nm gumarabic polysaccha-ride Matrix (Gara) particles final diameter of 189nm Final diameter 225 nm after PEI addition |
Polyethyleneimine (PEI) | -------- | Surface modification of carboxyl-bearing Gara nano particles with PEI | -------- | Chertok et al., 2010 |
| Fe3O4 |
Final diameter of (140-190 nm) |
Aminated, cross-linked starch and aminosilane coated Fe3O4 modified with PEG | To ensure that cross-linked starch particles was functionally similar to aminosilane coated particles, starch particles were covalently strengthened and aminated with concentrated ammonia to form aminated-precursor (DN). PEG is then linked to aminated precursors, DN and aminosilane particles with N-Hydroxysuccinimide (NHS) chemistry. | Cole et al., 2011 | ||
| Fe3O4 | Core diameter of 4-10 nm |
PVA and PVA with partially exchanged carboxyl groups. |
---- | ----- | Lee et al., 1996 | |
| Fe3O4 | Core diameter of 10 nm | PVA matrix | --- | the films of 200 mm depth and different concentrations of iron oxide particles in the PVA matrix. | ---- | Novakova et al., 2003 |
| Fe3O4 |
Core diameter of 5–10 nm Final diameter of 108-155 nm |
PVA | ---- | core-shell, all iron-oxide particles surrounded by a layer of PVA polymer. | ---- | Qui & Winnik, 2000 |
| γ-Fe203 |
Core diameter of 14, 19 and 43 nm Final particles are of diameter 43 nm |
PNIPAM | doxorubicin | MNP cluster is coated with PNIPAM and the nanoparticl is dehydrated. Core shell morphology is achieved with dispersion free-radical polymerization | Thermoresponsive | Purushotham et al., 2009 |
| Fe3O4 | core diameter of 13 nm | PNIPAM | doxorubicin | Core shell morphology by dispersion polymerization where drug loaded PNIPAM shell contains magnetite clusters. | Thermoresponsive | Purushotham et al., 2010 |
| Fe3O4 |
Core diameter of 11.21 nm Final particles are of diameter less than 250 µm |
PMMA |
fluorescein isothiocyanate (FITC) |
Thermoresponsive | Urbina et al., 2008 | |
| γ-Fe203 |
Core diameter of 20 nm Final particles are of diameter 400 nm |
carbon | doxorubicin | Drug is released form the surface of on-coated or partially coated magnetic particles |
released from the surface of our particles at a slow rate via desorption |
Ibarra et al., 2007 |
| Fe3O4 | Final particles are of diameter ~10–20 nm |
poly[aniline-co-sodium N-(1-onebutyric acid)] aniline (SPAnNa) |
1,3-bis(2-chloroethyl)-1- nitrosourea |
Microcapsule nanoparticles are encapsulated during the aggregation, forming the Fe3O4/SPAnH nanoparticles | Ultrasound and externally applied magnetic field. | Chen et al., 2010 |
| Fe3O4 |
Core diameter of 8 nm Final particles of diameter 5.2 µm |
PEs: poly(styrene sulfonate) (PSS, Mw~70000) and poly(allylamine hydrochloride) (PAH, Mw~50000). |
Melamine formaldehyde microparticle is coated with polyelectrolytes (PE) in a layer-by-layer (LbL) assembly by solvent controlled precipitation of PE. The core is then dissolved and nanoparticles are infiltrated into the capsule core. | Gaponik et al., 2004 | ||
| Fe3O4 | Final particle diameter of 300–1300 nm |
polystyrene |
Similar technique to abovementioned method. | Madani et al., 2011 | ||
| Fe3O4 |
Core diameter of 13 nm Final particle diameter of 3 µm |
poly(sodium 4-styrenesulfonate) (PSS) and poly(allylamine hydrochloride) (PAH) |
Dye |
Similar technique to abovementioned method. |
Magnetic heating | Katagiri et al., 2010 |
| Fe3O4 |
Core diameter of 20 nm Final particle diameter of 2.82 µm |
(PDDA/PSS)2/PDDA |
Dye | Similar technique to abovementioned method. | Magnetic heating | Katagiri et al., 2011 |
|
Fe3O4 and γ-Fe203 |
Fe3O4 and γ-Fe203 core diameters of 9.5 and 4.3 nm, respectively |
Ca alginate beads | ----- | The nanoparticles were entrapped in Ca alginate beads, “egg-box like” structure of Ca alginate | ------- | Finotelli et al., 2005 |
| Fe3O4 | Particle diameter of 58 nm | NP aggragates in humic acid (HA) | --- | HA adsorbs onto magnetite particles | ------ | Hu et al., 2010 |
| Fe3O4 | Final particle diameter of 7.5 nm | amino silane( 3-aminopropyl triethoxysilane) | --- |
nearly monolayer coating of amino silane on the magnetite particle surface |
--- | Ma et al., 2003 |
| Fe3O4 |
Core particle diameter of 10–15 nm Final particle diameter of 400±80 nm |
poly-L-lysine hydrochloride (PLL), poly-L-glutamic acid (PGA) | DNA |
layer-by-layer (LbL) assembly on polycarbonate templates with subsequent removal of these templates. In the inner surface of polycarbonate templates, first poly-L-lysine hydrochloride (PLL) and poly-L-glutamic acid (PGA) are absorbed linking by electrostatic interactions as a polyelectrolyte layer. Then, multi polyelectrolyte layers are assembled on polycarbonate membrane and Fe3O4 nanoparticles are linked to PLL layer as Fe3O4/PLL bilayers. |
----------- | He et al., 2008 |
| γ-Fe203 |
Core diameters of 12 nm. Final particle diameter of 35 nm (PEI) and 46 nm (PEI plus PEO-PGA) |
Poly(ethylene imine) and Poly(ethylene oxide)-block-poly(glutamic acid) |
--- | MNPs stabilized with polymers in two layer-by-layer deposition steps. | ---- | Thunemann et al., 2006 |
| Fe3O4 | Core diameter of 12 nm |
aminosilane coating |
--- | ---- | --- | Maier-Hauff et al., 2011 |
|
Fe3O4 and γ-Fe203 |
Core diameters of 10 nm Final particle diameter of 96 ±15 nm |
poly(ethylene glycol) (PEG) | doxorubicin | one-pot synthesis of colloids of SPION-DOX-PEG particles, PEG shell reduces the access of cellular enzymes to the drug-particle linkage and thus limits and/or delays the anticancer effect. |
specific release mechanism for drug delivery is enzymatic cleavage, however the PEG shell seems to reduce the access of cellular enzymes to the drug-particle linkage and thus limits and/or delays the anticancer effect. |
Shkilnyy et al., 2010 |
本文综述了磁性纳米颗粒在药物传递中的应用。磁性纳米颗粒由于其生物相容性、低毒性和在磁场作用下可操纵的能力而引起了人们的极大兴趣。这些特殊的性质允许它们被用作药物载体,通过直接将药物附着在颗粒上,或者通常通过使用天然或合成聚合物来帮助携带药物并将磁性颗粒嵌入聚合物基质中。已经探索了几种类型的药物和涂层作为药物载体,表1总结了非常有限的选择。这些颗粒表面修饰的便利性为靶向部分附着在颗粒表面提供了机会,从而促进了靶向。磁性纳米颗粒的靶向主要是在施加外部磁场的情况下进行的,该磁场作为一种外力将颗粒定位在体内所需的区域。一旦磁性粒子靠近肿瘤,对它们施加交变磁场,就会导致介质温度上升到42摄氏度,这是热疗所需的温度,热疗是与化疗和放疗一起进行的补充治疗。我们相信,在不久的将来,这些迷人的粒子将在现有的基础上获得更大的成功,并找到进一步的潜在应用。
参考文献
1. AlexiouC.TietzeR.SchreiberE.JurgonsR.RichterH.TrahmsL.RahnH.OdenbachS.LyerS.2011Cancer therapy with drug loaded magnetic nanoparticles-magnetic drug targeting, Journal of Magnetism and Magnetic Materials, 32314041407
2. AlphandéryE.GuyotF.ChebbiI.2012Preparation of chains of magnetosomes, isolated from Magnetospirillum magneticum strain AMB-1 magnetotactic bacteria, yielding efficient treatment of tumors using magnetic hyperthermia, International Journal of Pharmaceutics, 434444452
3. ArrueboM.GalanM.NavascuesN.TellezC.MarquinaC.IbarraM. R.SantamariaJ.2006Development of Magnetic Nanostructured Silica-Based Materials as Potential Vectors for Drug-Delivery Applications, Chemistry of Materials, 1819111919
4. AuffanM.DecomeL.Ros,eJ.OrsiereT.De MeoM.BrioisV.2006In vitro interactions between DMSA-coated maghemite nanoparticles and human fibroblasts: a physicochemical and cyto-genotoxical study, Environmental Science & Technology, 4043674373
5. BabaD.SeikoY.NakanishiT.ZhangH.ArakakiA.MatsunagaT.OsakaT.2012Effect of magnetite nanoparticles on living rate of MCF-7 human breast cancer cells, Colloids Surf B Biointerfaces, 95254257
6. BaeJ.E.HuhM.I.RyuB.K.DoJ.Y.JinS.U.MoonM.J.JungJ.C.ChangY.KimE.ChiS.G.LeeG.H.ChaeK.S.2011The effect of static magnetic fields on the aggregation and cytotoxicity of magnetic nanoparticles, Biomaterials, 3294019414
7. BanerjeeS. S.ChenD.H.2009Cyclodextrin-conjugated nanocarrier for magnetically guided delivery of hydrophobic drugs, Journal of Nanoparticle Research, 1120712078
8. BerryC. C.WellsS.CharlesS.CurtisA. S.2003Dextran and albumin derivatised iron oxide nanoparticles: influence on fibroblasts in vitro, Biomaterials, 24455457
9. BolfariniG. C.Siqueira-MouraM. P.DemetsG. J. F.MoraisP. C.TedescoA. C.2012In vitro evaluation of combined hyperthermia and photodynamic effects using magnetoliposomes loaded with cucurbit[7]uril zinc phthalocyanine complex on melanoma, Journal of Photochemistry and Photobiology B: Biology, DOI:10.1016/j.jphotobiol.2012.05.009,impress.
10. CaoH.HeJ.DengL.GaoX.2009Fabrication of cyclodextrin-functionalized superparamagnetic Fe3O4/amino-silane core-shell nanoparticles via layer-by-layer method, Applied Surface Science, 25579747980
11. CaruntuD.CaruntuG.O’ConnorC. J.2007Magnetic properties of variable-sized Fe3O4 nanoparticles synthesized from non-aqueous homogeneous solutions of polyols, Journal of Physics D: Applied Physics, 4058015809
12. ChanW. C. W.MaxwellD. J.GaoX. H.BaileyR. E.HanM. Y.NieS. M.2002Luminescent QDs for multiplexed biological detection and imaging, Current Opinion in Biotechnology, 134046
13. ChangE.AlexanderH. R.LibuttiS. K.HurstR.ZhaiS.FiggW. D.BartlettD. L.2001Laparoscopic continuous hyperthermic peritoneal perfusion, Journal of the American College of Surgeons, 193225229
14. ChenA.Z.LinX.F.WangS.B.LiL.LiuY.G.YeL.WangG.Y.2012Biological evaluation of Fe3O4-poly(l-lactide)-poly(ethylene glycol)-poly(l-lactide) magnetic microspheres prepared in supercritical CO2, Toxicology Letters, 2127582
15. ChenB.ZhangH.ZhaiC.DuN.SunC.XueJ.YangD.HuangH.ZhangB.XiecQ.WuY.2010Carbon nanotube-based magnetic-fluorescent nanohybrids as highly efficient contrast agents for multimodal cellular imaging, Journal of Materials Chemistry, 2098959902
16. ChenD.JiangM.LiN.GuH.XuQ.GeJ.XiaX.LuJ.2010Modification of magnetic silica/iron oxide nanocomposites with fluorescent polymethacrylic acid for cancer targeting and drug delivery, Journal of Materials Chemistry, 2064226429
17. ChenF.H.ZhangL.M.ChenQ.T.ZhangY.ZhangZ.J.2010Synthesis of a novel magnetic drug delivery system composed of doxorubicin-conjugated Fe3O4 nanoparticle cores and a PEG-functionalized porous silica Shell, Chemical Communications, 4686338635
18. ChenP.Y.LiuH.L.HuaM.Y.YangH.W.HuangC.Y.ChuP.C.LyuL.A.Tseng-CI.FengL.Y.TsaiH.C.ChenS.M.LuY.J.WangJ.J.YenT.C.MaY.H.WuT.ChenJ.P.ChuangJ.I.ShinJ.W.HsuehC.WeiK.C.2010Novel magnetic/ultrasound focusing system enhances nanoparticle drug delivery for glioma treatment, Neuro-Oncology, 1210501060
19. ChertokB.DavidA. E.YangV. C.2010Polyethyleneimine-modified iron oxide nanoparticles for brain tumor drug delivery using magnetic targeting and intra-carotid administration, Biomaterials, 3163176324
20. ChertokB.MoffatB. A.DavidA. E.YuF.BergemannC.RossB. D.YangV. C.2008Iron Oxide Nanoparticles as a Drug Delivery Vehicle for MRI Monitored Magnetic Targeting of Brain Tumors, Biomaterials, 29487496
21. ColeA. J.DavidA. E.WangJ.GalbánC. J.HillH. L.YangV. C.2011Polyethylene glycol modified, cross-linked starch-coated iron oxide nanoparticles for enhanced magnetic tumor targeting, Biomaterials, 3221832193
22. ColeA. J.DavidA. E.WangJ.GalbánC. J.YangV. C.2011Magnetic brain tumor targeting and biodistribution of long-circulating PEG-modified, cross-linked starch-coated iron oxide nanoparticles, Biomaterials, 3262916301
23. ColeA. J.YangV. C.DavidA. E.2011Cancer theranostics: the rise of targeted magnetic nanoparticles, Trends in Biotechnology, 29323332
24. CuiM.WangF.J.ShaoZ.Q.LuF.S.WangW.J.2011Influence of DS of CMC on morphology and performance of magnetic microcapsules, Cellulose, 1812651271
25. De ChâtelP. F.NándoriI.HaklJ.MészárosS.VadK.2009Magnetic particle hyperthermia: Néel relaxation in magnetic nanoparticles under circularly polarized field, Journal of Physics: Condensed Matter, 21124202124210
26. DingJ.TaoK.LiJ.SongS.SunK.2010Cell-specific cytotoxicity of dextran-stabilized magnetite nanoparticles, Colloids and Surfaces B: Biointerfaces, 79184190
27. DingG.GuoY.LvY.LiuX.XuL.ZhangX.2012A double-targeted magnetic nanocarrier with potential application in hydrophobic drug delivery, Colloids and Surfaces B: Biointerfaces, 916876
28. DutzS.KetteringM.HilgerI.MüllerR.ZeisbergerM.2011Magnetic multicore nanoparticles for hyperthermia--influence of particle immobilization in tumor tissue on magnetic properties, Nanotechnology, 22265102265109
29. ElsherbiniA. A.SaberM.AggagM.El -ShahawyA.ShokierH. A.2011Magnetic nanoparticle-induced hyperthermia treatment under magnetic resonance imaging, Magnetic Resonance Imaging, 29272280
30. FalkM. H.IsselsR. D.2001Hyperthermia in oncology, International Journal of Hyperthermia, 17118
31. FeldmanA. L.LibuttiS. K.PingpankJ. F.BartlettD. L.BeresnevT. H.MavroukakisS. M.SteinbergS. M.LiewehrD. J.KleinerD. E.AlexanderH. R.2003Analysis of Factors Associated with Outcome in Patients with Malignant Peritoneal Mesothelioma Undergoing Surgical Debulking and Intraperitoneal Chemotherapy, Journal of Clinical Oncology, 2145604567
32. FinotelliP. V.DaSilva. D.Sola-PennaM.RossiA. M.FarinaM.AndradeL. R.TakeuchiA. Y.Rocha-LeaoM. H.2010Microcapsules of alginate/chitosan containing magnetic nanoparticles for controlled release of insulin, Colloids and Surfaces B: Biointerfaces, 81206211
33. FinotelliP. V.SampaioD. A.MoralesM. A.RossiA. M.Rocha-LeãoM. H.2005Ca Alginate as Scaffold for Iron Oxide Nanoparticles Synthesis, 2nd Mercosur Congress on Chemical Engineering, 4th Mercosur Congress on Process Systems Engineering, Rio de Janeiro- Brazil.
34. ForbesZ. G.YellenB. B.BarbeeK. A.FriedmanG.2003An Approach to Targeted Drug Delivery Based on Uniform Magnetic Fields, IEEE Transactions on Magnetics, 3933723377
35. GaponikN.RadtchenkoI. L.SukhorukovG. B.RogachA. L.2004Luminescent Polymer Microcapsules Addressable by a Magnetic Field, Langmuir, 2014491452
36. GitterK.OdenbachS.2011Experimental investigations on a branched tube model in magnetic drug targeting, Journal of Magnetism and Magnetic Materials, 32314131416
37. GitterK.OdenbachS.2011Quantitative targeting maps based on experimental investigations for a branched tube model in magnetic drug targeting, Journal of Magnetism and Magnetic Materials, 32330383042
38. GlöcklG.HergtR.ZeisbergerM.DutzS.NagelS.WeitschiesW.2006The effect of field parameters, nanoparticle properties and immobilization on the specific heating power in magnetic particle hyperthermia, Journal of Physics: Condensed Matter, 18S2935S2949
39. Gonzales-WeimullerM.ZeisbergerM.KrishnanK. M.2009Size-dependant heating rates of ironoxide nanoparticles for magnetic fluid hyperthermia, Journal of Magnetism and Magnetic Materials, 32119471950
40. GoyaG. F.LimaE.Jr ArelaroA. D.TorresT.RechenbergH. R.RossiL.MarquinaC.IbarraM. R.2008Magnetic Hyperthermia with Fe3O4 Nanoparticles: The Influence of Particle Size on Energy Absorption, IEEE Transactions on Magnetics, 4444444447
41. HäfeliU. A.RiffleJ. S.Carmichael-BaranauskasA.Harris-ShekhawatL.MarkF.DaileyJ. P.BardensteinD.2009Cell Uptake and in vitro Toxicity of Magnetic Nanoparticles Suitable for Drug Delivery, Molecular Pharmaceutics, 614171428
42. HayashiK.MaedaK.MoriyaM.SakamotoW.YogoT.2012In situ synthesis of cobalt ferrite nanoparticle / polymer hybrid from a mixed Fe-Co methacrylate for magnetic hyperthermia, Journal of Magnetism and Magnetic Materials, 32431583164
43. HeQ.TianY.CuiY.MöhwaldH.LiJ.2008Layer-by-layer assembly of magnetic polypeptide nanotubes as a DNA carrier, Journal of Materials Chemistry, 18748754
44. HergtR.DutzS.2007Magnetic particle hyperthermia-biophysical limitations of a visionary tumor therapy, Journal of Magnetism and Magnetic Materials, 311187192
45. HergtR.DutzS.MüllerR.ZeisbergerM.2006Magnetic particle hyperthermia: nanoparticle magnetism and materials development for cancer therapy, Journal of Physics: Condensed Matter, 18S2919S2934
46. HuJ.D.ZeviY.KouX.M.XiaoJ.WangX.J.JinY.2010Effect of dissolved organic matter on the stability of magnetite nanoparticles under different pH and ionic strength conditions, Science of the Total Environment, 40834773489
47. HuS.H.TsaiC.H.LiaoC.F.LiuD.M.ChenS.Y.2008Controlled Rupture of Magnetic Polyelectrolyte Microcapsules for Drug Delivery, Langmuir, 241181111818
48. HuaM.Y.LiuH.L.YangH.W.ChenP.Y.TsaiR.Y.HuangC.Y.TsengI.C.LyuL.A.MaC.C.TangH.J.YenT.C.WeiK.C.2011The effectiveness of a magnetic nanoparticle-based delivery system for BCNU in the treatment of gliomas, Biomaterials, 32516527
49. HuaM. Y.YangH. W.LiuH. L.TsaiR. Y.PangS. T.ChuangK. L.ChangY. S.HwangT. L.ChangY. H.ChuangH. C.ChuangC. K.2011Superhigh-magnetization nanocarrier as a doxorubicin delivery platform for magnetic targeting therapy, Biomaterials, 3289999010
50. HuangC.TangZ.ZhouY.ZhouX.JinY.LiD.YangY.ZhouS.2012Magnetic micelles as a potential platform for dual targeted drug delivery in cancer therapy, International Journal of Pharmaceutics, 429113122
51. HuongN. T.GiangL. T. K.BinhN. T.MinhL. Q.2009Surface modification of iron oxide nanoparticles and their conjunction with water soluble polymers for biomedical application, Journal of Physics: Conference Series, 187012046012051
52. IbarraM. R.Fernandez-PachecoR.ValdiviaJ. G.MarquinaC.GutierrezM.2007Magnetic Nanoparticle Complexes for Drug Delivery, and Implanted Magnets for Targeting, American Institute of Physics, 89899105
53. IchiyanagiaY.ShigeokaD.HirokiT.MashinoT.KimuraS.TomitakaA.UedaK.TakemuraY.2012Study on increase in temperature of Co-Ti ferrite nanoparticles for magnetic hyperthermia treatment, Thermochimica Acta, 532123126
54. JordanA.ScholzR.Maier-HauffK.van LandeghemF. K. H.WaldoefnerN.TeichgraeberU.PinkernelleJ.BruhnH.NeumannF.ThiesenB.vonDeimling. A.FelixR.2006The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma, Journal of Neuro-Oncology, 78714
55. JordanA.WustP.FählingH.JohnW.HinzA.FelixR.1993Inductive heating of ferrimagnetic particles and magnetic fluids: physical evaluation of their potential for hyperthermia, International Journal of Hyperthermia, 95168
56. JungC. W.JacobsP.1995Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil, Magnetic Resonance Imaging, 13661674
57. KallumadilM.TadaM.NakagawaT.AbeM.SouthernP.PankhurstQ. A.2009Suitability of commercial colloids for magnetic hyperthermia, Journal of Magnetism and Magnetic Materials, 32115091513
58. KappiyoorR.LiangruksaM.GangulyR.PuriI. K.2010The effects of magnetic nanoparticle properties on magnetic fluid Hyperthermia, Journal of Applied Physics, 108094702094708
59. KarlssonH. L.CronholmP.GustafssonJ.MollerL.2008Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes, Chemical Research in Toxicology, 2117261732
60. KarlssonH. L.GustafssonJ.CronholmP.MollerL.2009Size dependent toxicity of metal oxide particles--a comparison between nano- and micrometer size, Toxicology Letters, 188112118
61. KatagiriK.ImaiY.KoumotoK.2011Variable on-demand release function of magnetoresponsive hybrid capsules, Journal of Colloid and Interface Science, 361109114
62. KatagiriK.NakamuraM.KoumotoK.2010Magnetoresponsive Smart Capsules Formed with Polyelectrolytes, Lipid Bilayers and Magnetic Nanoparticles, American Chemical Society, 2768773
63. KoppoluB.RahimiM.NattamaS.WadajkarA.NguyenK. T.2010Development of multiple-layer polymeric particles for targeted and controlled drug delivery, Nanomedicine: Nanotechnology, Biology, and Medicine, 6355361
64. KrukemeyerM. G.KrennV.JakobsM.WagnerW.2012Mitoxantrone-iron oxide biodistribution in blood, tumor, spleen and liver-magnetic nanoparticles in cancer treatment, Journal of Surgical Research, 1753543
65. KrupskayaY.MahnC.ParameswaranA.TaylorA.KramerK.HampelS.LeonhardtA.RitschelM.BüchnerB.KlingelerR.2009Magnetic study of iron-containing carbonnanotubes: Feasibility for Magnetic hyperthermia, Journal of Magnetism and Magnetic Materials, 32140674071
66. KulshresthaP.GogoaM.BahadurD.BanerjeeR.2012In vitro application of paclitaxel loaded magnetoliposomes for combined chemotherapy and hyperthermia, Colloids and Surfaces B: Biointerfaces, 9617
67. KumarC. S. S. R.MohammadF.2011Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery, Advanced Drug Delivery Reviews, 63789808
68. KuznetsovO. A.BrusentsovN. A.KuznetsovA. A.YurchenkoN. Y.OsipovN. E.BayburtskiyF. S.1999Correlation of the coagulation rates and toxicity of biocompatible ferromagnetic microparticles, Journal of Magnetism and Magnetic Materials, 1948389
69. Le RenardP.E.JordanO.FaesA.Petri-FinkA.HofmannH.RüfenachtD.BosmanF.BucheggerF.DoelkerE.2010The in vivo performance of magnetic particle-loaded injectable, in situ gelling, carriers for the delivery of local hyperthermia, Biomaterials, 31691705
70. LeeE.H.KimC.Y.ChoaY.H.2012Magnetite nanoparticles dispersed within nanoporous aerogels for hyperthermia Application, Current Applied Physics, 2012, doi:10.1016/j.cap.2012.02.017,impress.
71. LeeJ.IsobeT.SennaM.1996Magnetic properties of ultrafine magnetite particles and their slurries prepared via in-situ precipitation, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 109121127
72. LeeJ.H.SchneiderB.JordanK.E.LiuW.FrankJ. A.2008Synthesis of complexable fluorescent superparamagnetic iron oxide nanoparticles (FL SPIONs) and its cell labeling for clinical application, Advanced Material, 2025122516
73. LeeK.J.AnJ.H.ShinJ.S.KimD.H.YooH.S.ChoC.K.2011Biostability of γ-Fe2O3 nanoparticles Evaluated using an in vitro cytotoxicity assays on various tumor cell lines, Current Applied Physics, 11467471
74. LiL.ChenD.ZhangY.DengZ.RenX.MengX.TangF.RenJ.ZhangL.2007Magnetic and fluorescent multifunctional chitosan nanoparticles as a smart drug delivery system, Nanotechnology, 18405102405108
75. LiaoC.SunQ.LiangB.ShenJ.ShuaiX.2011Targeting EGFR-overexpressing tumor cells using cetuximab-immunomicelles loaded with doxorubicin and superparamagnetic iron oxide, European Journal of Radiology, 80699705
76. LiuR.-t.LiuJ.TongJ.-q.TangT.KongW.C.WangX.-w.LiY.TangJ.-t.2012Heating effect and biocompatibility of bacterial magnetosomes as potential materials used in magnetic fluid hyperthermia, Progress in NaturalScience: Materials International, 223139
77. MaJ.ChenD.TianY.TaoK.2000Toxicity of Magnetic Albumin Microspheres Bearing Adriamycin, Journal of Tongji Medical University, 202261262
78. MaM.ZhangY.YuW.ShenH.-y.ZhangH.-q.GuN.2003Preparation and characterization of magnetite nanoparticles coated by amino silane, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 212219226
79. MadaniM.Sharifi-SanjaniN.Faridi-MajidiR.2011Magnetic polystyrene nanocapsules with core-shell morphology obtained by emulsifier-free miniemulsion polymerization, Polymer Science, Ser. A, 53143148
80. MahmoudiM.SantS.WangB.LaurentS.SenT.2011Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy, Advanced Drug Delivery Reviews, 632446
81. MahmoudiM.SimchiA.ImaniM.MilaniA. S.StroeveP.2009An in vitro study of bare and poly(ethylene glycol)-co-fumarate coated superparamagnetic iron oxide nanoparticles: a new toxicity identification procedure, Nanotechnology, 20225104
82. MahmoudiM.SimchiA.ImaniM.ShokrgozarM. A.MilaniA. S.HäfeliU. O.StroeveP.2010A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles, Colloids and Surfaces B: Biointerfaces, 75300309
83. MahmoudiM.SimchiA.MilaniA. S.StroeveP.2009Cell toxicity of superparamagnetic iron oxide nanoparticles, Journal of Colloid and Interface Science, 336510518
84. Maier-HauffK.UlrichF.NestlerD.NiehoffH.WustP.ThiesenB.OrawaH.BudachV.JordanA.2011Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme, Journal of Neuro-Oncology, 103317324
85. MangualJ. O.AvilesM. O.EbnerA. D.RitterJ. A.2011In vitro study of magnetic nanoparticles as the implant for implant assisted magnetic drug targeting, Journal of Magnetism and Magnetic Materials, 32319031908
86. Martín-SaavedraF. M.Ruíz-HernándezE.BoréA.ArcosD.Vallet-RegíM.VilaboaN.2010Magnetic mesoporous silica spheres for hyperthermia therapy, Acta Biomaterialia, 645224531
87. MeenachS. A.HiltJ. Z.AndersonK. W.2010Poly(ethylene glycol)-based magnetic hydrogel nanocomposites for hyperthermia cancer therapy, Acta Biomaterialia, 610391046
88. MishimaF.TakedaS.IzumiY.NishijimaS.2007Development of Magnetic Field Control for Magnetically Targeted Drug Delivery System Using a Superconducting Magnet, IEEE Transactions on Applied Superconductivity, 1723032306
89. MiyazakiT.MiyaokaA.IshidaE.LiZ.KawashitaM.HiraokaM.2012Preparation of ferromagnetic microcapsules for hyperthermia using water/oil emulsion as a reaction field, Materials Science and Engineering C, 32692696
90. MorozP.PardoeH.JonesS. K.StPierre. T. G.SongS.GrayB. N.2002Arterial embolization hyperthermia: hepatic iron particle distribution and its potential determination by magnetic resonance imaging, Physics in Medicine and Biology, 4715911602
91. MuldoonL. L.SandorM.PinkstonK. E.NeuweltE. A.2005Imaging, distribution, and toxicity of superparamagnetic iron oxide magnetic resonance nanoparticles in the rat brain and intracerebral tumor, Neurosurgery, 57785796
92. NovakovaA. A.LanchinskayaV.YuVolkovA. V.GendlerT. S.KiselevaT.YuMoskvinaM. A.ZezinS. B.2003Magnetic properties of polymer nanocomposites containing iron oxide nanoparticles, Journal of Magnetism and Magnetic Materials, 258354357
93. PanczykT.WarzochaT. P.CampP. J.2010A magnetically controlled molecular nanocontainer as a drug delivery system: The effects of carbon nanotube and magnetic nanoparticle parameters from monte carlo simulations, The Journal of Physical Chemistry C, 1142129921308
94. PardoeH.ClarkP. R.StPierre. T. G.MorozP.JonesS. K.2003A magnetic resonance imaging based method for measurement of tissue iron concentration in liver arterially embolized with ferromagnetic particles designed for magnetic hyperthermia treatment of tumors, Magnetic Resonance Imaging, 21483488
95. ParkJ.H.ImK.H.LeeS.H.KimD.H.LeeD.Y.LeeY.K.KimK.M.KimK.N.2005Preparation and characterization of magnetic chitosan particles for hyperthermia application, Journal of Magnetism and Magnetic Materials, 293328333
96. PisanicT. R.2ndBlackwellJ. D.ShubayevV. I.FinonesR. R.JinS.2007Nanotoxicity of iron oxide nanoparticle internalization in growing neurons, Biomaterials, 2825722581
97. PradhanP.GiriJ.RiekenF.KochC.MykhaylykO.DöblingerM.BanerjeeR.BahadurD.PlankC.2010Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy, Journal of Controlled Release, 142108121
98. PurushothamS.ChangP. E. J.RumpelH.KeeI. H. C.NgR. T. H.ChowP. K. H.Tan. C. K.RamanujanR. V.2009Thermoresponsive core-shell magnetic nanoparticles for combined modalities of cancer Therapy, Nanotechnology, 20305101305112
99. PurushothamS.RamanujanR. V.2010Thermoresponsive magnetic composite nanomaterials for multimodal cancer therapy, Acta Biomaterialia, 6502510
100. QuiX.-p.WinnikF.2000Preparation and characterization of PVA coated magnetic nanoparticles Chinese journal of polymer science, 18535539
101. RajuH. B.HuY.VedulaA.DubovyS. R.GoldbergJ. L.2011Evaluation of magnetic micro- and nanoparticle toxicity to ocular tissues, PLoS One, 6e17452e17463
102. RaynalI.PrigentP.PeyramaureS.NajidA.RebuzziC.CorotC.2004Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10, Investigative Radiology, 395663
103. RiveraG. P.HuhnD.del MercatoL. L.SasseD.ParakW. J.2010Nanopharmacy: Inorganic nanoscale devices as vectors and active compounds, Pharmacological Research, 62115125
104. Ruiz-HernandezE.BaezaA.Vallet-RegiM.2011Smart Drug Delivery through DNA/Magnetic Nanoparticle Gates, American Chemical Society, 512591266
105. SaavedraF. M.Ruíz-HernándezE.BoréA.ArcosD.Vallet-RegíM.VilaboaN.2010Magnetic mesoporous silica spheres for hyperthermia therapy, Acta Biomaterialia, 645224531
106. SahuS. K.MaitiS.PramanikA.GhoshS. K.PramanikP.2012Controlling the thickness of polymeric shell on magnetic nanoparticles loaded with doxorubicin for targeted delivery and MRI contrast agent, Carbohydrate Polymers, 8725932604
107. SalloumM.MaR.ZhuL.2008An in-vivo experimental study of temperature elevations in animal tissue during magnetic nanoparticle hyperthermia, International Journal of Hyperthermia, 24589601
108. SalloumM.MaR.ZhuL.2009Enhancement in treatment planning for magnetic nanoparticle hyperthermia: optimization of the heat absorption pattern, International Journal of Hyperthermia, 25309321
109. chweigerC.PietzonkaC.HeverhagenJ.KisselT.2011Novel magnetic iron oxide nanoparticles coated with poly(ethylene imine)-g-poly(ethylene glycol) for potential biomedical application: Synthesis, stability, cytotoxicity and MR imaging, International Journal of Pharmaceutics 408130137
110. SharifiI.ShokrollahiH.AmiriS.2012Ferrite-based magnetic nanofluids used in hyperthermia applications, Journal of Magnetism and Magnetic Materials, 324903915
111. ShenS.RenJ.ChenJ.LuX.DengC.JiangX.2011Development of magnetic multiwalled carbon nanotubes combined with near-infrared radiation-assisted desorption for the determination of tissue distribution of doxorubicin liposome injects in rats, Journal of Chromatography A, 121846194626
112. ShkilnyyA.MunnierE.HerveK.SouceM.BenoitR.Cohen-JonathanS.LimeletteP.SaboungiM.L.DuboisP.ChourpaI.2010Synthesis and Evaluation of Novel Biocompatible Super-paramagnetic Iron Oxide Nanoparticles as Magnetic Anticancer Drug Carrier and Fluorescence Active Label, Journal of Physical Chemistry C, 11458505858
113. ShundoC.ZhangH.NakanishiT.OsakaT.2012Cytotoxicity evaluation of magnetite (Fe3O4) nanoparticles in mouse embryonic stem cells, Colloids and Surfaces B: Biointerfaces, 97221225
114. SiebenS.BergemannC.LuKbbe. A.BrockmannB.RescheleitD.2001Comparison of different particles and methods for magnetic isolation of circulating tumor cells, Journal of Magnetism and Magnetic Materials, 225175179
115. SimioniA. R.PrimoF. L.RodriguesM. M. A.LacavaZ. G. M.MoraisP. C.TedescoA. C.2007Preparation, Characterization and in vitro Toxicity Test of Magnetic Nanoparticle-Based Drug Delivery System to Hyperthermia of Biological Tissues, IEEE Transactions on Magnetics, 4324592461
116. SinghN.JenkinsG. J. S.NelsonB. C.MarquisB. J.MaffeisT. G. G.BrownA. P.WilliamsP. M.WrightC. J.DoakS. H.2012The role of iron redox state in the genotoxicity of ultrafine superparamagnetic iron oxide nanoparticles, Biomaterials, 33163170
117. SkumielA.2006Suitability of water based magnetic fluid with CoFe2O4 particles in hyperthermia, Journal of Magnetism and Magnetic Materials, 3078590
118. SuW.WangH.WangS.LiaoZ.KangS.PengY.HanL.ChangJ.2012PEG/RGD-modified magnetic polymeric liposomes for controlled drug release and tumor cell targeting, International Journal of Pharmaceutics, 426170181
119. SunY.ChenZ.YangX.HuangP.ZhouX.DuX.2009Magnetic chitosan nanoparticles as a drug delivery system for targeting photodynamic therapy, Nanotechnology, 20135102135110
120. ThunemannA. F.SchuttD.KaufnerL.PisonU.MohwaldH.2006Maghemite Nanoparticles Protectively Coated with Poly(ethylene imine) and Poly(ethylene oxide)-block-poly(glutamic acid), Langmuir, 2223512357
121. TungW. L.HuS. H.LiuD. M.2011Synthesis of nanocarriers with remote magnetic drug release control and enhanced drug delivery for intracellular targeting of cancer cells, Acta Biomaterialia, 728732882
122. UrbinaM. C.ZinovevaS.MillerT.SabliovC. M.MonroeW. T.KumarC. S. S. R.2008Investigation of Magnetic Nanoparticle-Polymer Composites for Multiple-controlled Drug Delivery, The Journal of Physical Chemistry C, 1121110211108
123. Van der ZeeJ.2002Heating the patient: a promising approach?, Annals of Oncology, 1311731184
124. VeisehO.GunnJ. W.ZhangM.2010Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging, Advanced Drug Delivery Reviews, 62284304
125. VermisoglouE. C.PilatosG.RomanosG. E.DevlinE.KanellopoulosN. K.KaranikolosG. N.2011Magnetic carbon nanotubes with particle-free surfaces and high drug loading capacity, Nanotechnology, 22355602355612
126. VoitW.KimD. K.ZapkaW.MuhammedM.RaoK. V.2001Magnetic behavior of coated superparamagnetic iron oxide nanoparticles in ferrofluids, Materials Research Society Symposium Proceedings, 676Y7Y7.8.6.
127. WangH.WangS.LiaoZ.ZhaoP.SuW.NiuR.ChangJ.2012Folate-targeting magnetic core-shell nanocarriers for selective drug release and imaging, International Journal of Pharmaceutics, 430342349
128. WangL.NeohK. G.KangE.T.ShuterB.2011Multifunctional polyglycerol-grafted Fe3O4@SiO2 nanoparticles for targeting ovarian cancer cells, Biomaterials, 3221662173
129. WinerJ. L.LiuC. Y.ApuzzoM. L. J.2011The Use of Nanoparticles as Contrast Media in Neuroimaging: A Statement on Toxicity, World Neurosurgery, DOI:10.1016/j.wneu.2011.08.013,impress.
130. WuH.LiuG.WangX.ZhangJ.ChenY.ShiJ.YangH.HuaH.YangS.2011Solvothermal synthesis of cobalt ferrite nanoparticles loaded on multiwalled carbon nanotubes for magnetic resonance imaging and drug delivery, Acta Biomaterialia, 734963504
131. WustP.HildebrandtB.SreenivasaG.RauB.GellermannJ.RiessH.FelixR.SchlagP. M.2002Hyperthermia in combined treatment of cancer, The Lancet Oncology, 3487497
132. WustP.HildebrandtB.SreenivasaG.RauB.GellermannJ.RiessH.FelixR.SchlagP. M.2002Hyperthermia in combined treatment of cancer. The Lancet Oncology 3487497
133. YallapuM. M.OthmanS. F.CurtisE. T.GuptaB. K.JaggiM.ChauhanS. C.2011Multi-functional magnetic nanoparticles for magnetic resonance imaging and cancer therapy, Biomaterials, 3218901905
134. YangF.FuD. L.LongJ.NiQ. X.2008Magnetic lymphatic targeting drug delivery system using carbon nanotubes, Medical Hypotheses, 70765767
135. YangF.JinC.YangD.JiangY.LiJ.Di Y.HuJ.WangC.NiQ.FuD.2011Magnetic functionalised carbon nanotubes as drug vehicles for cancer lymph node metastasis treatment, European Journal of Cancer, 4718731882
136. YangJ.LeeH.HyungW.ParkS.B.HaamS.2006Magnetic PECA nanoparticles as drug carriers for targeted delivery: Synthesis and release characteristics, Journal of Microencapsulation, 23203212
137. YangQ.LiangJ.HanH.2009Probing the Interaction of Magnetic Iron Oxide Nanoparticles with Bovine Serum Albumin by Spectroscopic Techniques, The Journal of Physical Chemistry B, 1131045410458
138. YangX.HongH.GrailerJ. J.RowlandI. J.JavadiA.HurleyS. A.XiaoY.YangY.ZhangY.NicklesR. J.CaiW.SteeberD. A.GongS.2011cRGD-functionalized, DOX-conjugated, and ⁶⁴Cu-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and PET/MR imaging, Biomaterials, 3241514160
139. YangY.JiangJ. S.DuB.GanZ. F.QianM.ZhangP.2009Preparation and properties of a novel drug delivery system with both magnetic and biomolecular targeting, Journal of Materials Science: Materials in Medicine, 20301307
140. YaoA.AiF.WangD.HuangW.ZhangX.2009Synthesis, characterization and in vitro cytotoxicity of self-regulating magnetic implant material for hyperthermia application, Materials Science and Engineering C, 2925252529
141. YellenB. B.ForbesZ. G.HalversonD. S.FridmanG.BarbeeK. A.ChornyM.2005 Targeted drug delivery to magnetic implants for therapeutic applications, Journal of Magnetism and Magnetic Materials, Vol.293, pp.647-654.
142. YildirimerL.ThanhN. T. K.LoizidouM.SeifalianA. M.2011Toxicological considerations of clinically applicable Nanoparticles, Nano Today, 6585607
143. ZefengX.GuobinW.KaixiongT.JianxingL.YuanT.2005Preparation and acute toxicology of nano-magnetic ferrofluid, Journal of Huazhong University of Science and Technology-- Medical Sciences, 255961
144. ZhangL.ZhangF.WangY.S.SunY.L.DongW.F.SongJ.F.HuoQ.S.SunH.B.2011Magnetic colloidosomes fabricated by Fe3O4-SiO2 hetero-nanorods, Soft Matter, 773757381
145. ZhangY. Q.LiL. L.TangF.RenJ.2006Controlled Drug Delivery System Based on Magnetic Hollow Spheres/Polyelectrolyte Multilayer Core-Shell Structure, Journal of Nanoscience and Nanotechnology, 632103214
146. ZhouH.TaoK.DingJ.ZhangZ.SunK.ShiW.2011A general approach for providing nanoparticles water-dispersibility by grinding with poly (ethylene glycol). Colloids and Surfaces A: Physicochemical and Engineering Aspects 2011; 3891826
- 上一篇:铁磁流体的用途 2023/11/4
- 下一篇:儿茶酚胺及其代谢物检测:部分肿瘤的检测标记物 2023/11/3
